Chemical Properties
solid
Uses
As a dye: Colour Index vol. 2 (3rd ed., 1971) p 2223. As analytical reagent in detection of Mg.
Preparation
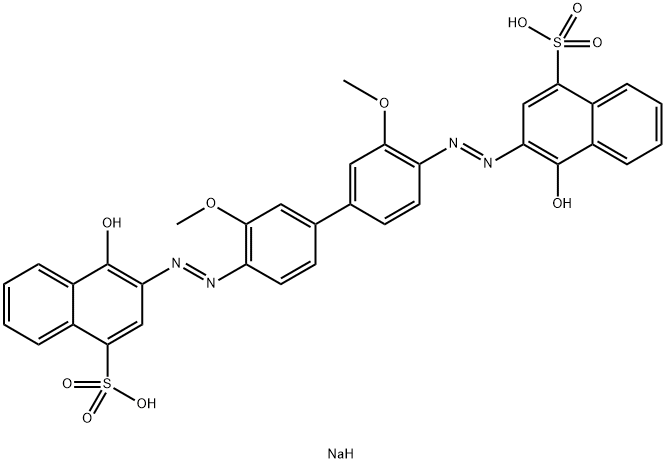
3,3′-Dimethoxylbenzidine ?double nitride, and 4-Hydroxynaphthalene-1-sulfonic acid (2 Moore) coupled.
General Description
Bluish-black powder.
Air & Water Reactions
Azo dyes can be explosive when suspended in air at specific concentrations. Slightly soluble in water.
Reactivity Profile
BENZOAZURINE is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
Fire Hazard
Flash point data are not available for BENZOAZURINE, but BENZOAZURINE is probably combustible.
Properties and Applications
blue. Soluble in water for purple red to blue, soluble in soluble fiber element, soluble in ethanol, insoluble in other organic solvents. The strong sulfuric acid for blue, blue for red after diluted. The dye solution to join strong hydrochloric acid for purple, have precipitation; Add thick sodium hydroxide solution for red light purple.
|
Standard
|
Acid Resistance
|
Alkali Resistance
|
Light Fastness
|
Soaping
|
Water
|
|
Fading
|
Stain
|
Fading
|
Stain
|
|
ISO
|
4-5
|
2
|
1
|
1-2
|
|
1-2
|
|
|
AATCC
|
5
|
1
|
1-2
|
1
|
|
1
|
|