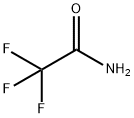
Chemical Properties
white to pale yellow or beige crystalline powder
Uses
2,2,2-Trifluoroacetamide used in a convenient alternative to the Gabriel synthesis of primary amines from halides by N-alkylation followed by cleavage of the readily-hydrolyzed trifluoroacetyl group and for improved N-alkylation under phase-transfer conditions allowing successive alkylation with different alkyl groups.
Application
Trifluoroacetamide was used as probe for determination of membrane potential and extra/intracellular volume of erythrocytes by fluorine-19 NMR studies.
Preparation
One-pot synthetic approach to produce trifluoroacetamide has been developed using an electrochemical method with the B12 complex as a catalyst under mild conditions, in open air at room temperature. Thirty examples of trifluoroacetamide were synthesized from 1,1,1-trichloro-2,2,2-trifluoroethane (CFC-113a) in moderate to good yields. This user-friendly strategy is compatible with a broad range of trifluoroacetamide syntheses.
10.1142/S1088424621500292
General Description
Trifluoroacetamide is a good quencher of tryptophan fluorescence.