Uses
1-Bromo-4-chloro-2-nitrobenzene is used in the synthesis of diindolocarbazoles used in the synthesis of ladder oligo(p-aniline)s useful in organic electronics. Also used in the synthesis of novel benzenesulfonamides for the discovery of potent cell cycle inhibitors.
Description
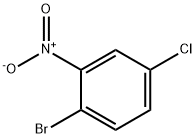
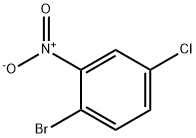
2-Bromo-5-chloronitrobenzene is an organic compound, the molecular formula is C
6H
3BrClNO
2. It is white to off-white crystal powder.
Uses
2-bromo-4-methylpropiophenone employed as an important intermediate for raw material for organic synthesis, agrochemical, pharmaceutical and dyestuff field. Specifically, this chemical can act as the intermediate in the synthesis of 4-methyl methcathinone HCl (mephedrone HCl or 4-MMC HCl), which is used as the sample for development and validation of a presumptive color spot test method for the detection of piperazine analogues in seizing illicit materials.
Chemical Properties
white to light yellow crystal powder
Uses
1-Bromo-4-chloro-2-nitrobenzene may be used in chemical synthesis studies.
Synthesis
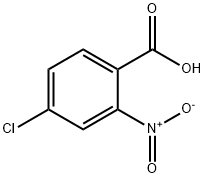
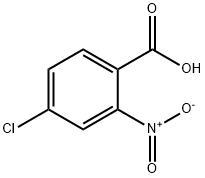
General procedure for the synthesis of 2-bromo-5-chloronitrobenzene from 4-chloro-2-nitrobenzoic acid: In a 25 mL round-bottomed flask, fitted with a Dimroth condenser (cooled to 10 °C), 4-chloro-2-nitrobenzoic acid (1.8 mmol), chloroisocyanuric acid ester, brominating agent and solvent (8 mL) were added. The reaction mixture was stirred and heated in an oil bath under fluorescent room light (FL) irradiation. Upon completion of the reaction, the cooled mixture was filtered through a short silica gel pad, washed with 1 M aqueous Na2SO3, dried over anhydrous Na2SO4, filtered and concentrated in vacuum to give 2-bromo-5-chloronitrobenzene. The product may contain 1-5% of 2-chloro-5-nitrobenzene as a by-product. The experimental results are detailed in Table 2. Table 2. Results of bromocarboxylation of nitroaromatic carboxylic acids
References
[1] Patent: WO2017/60906, 2017, A1. Location in patent: Paragraph 00122
[2] Journal of Organic Chemistry, 2016, vol. 81, # 7, p. 2794 - 2803
[3] Organic and Biomolecular Chemistry, 2018, vol. 16, # 30, p. 5416 - 5421