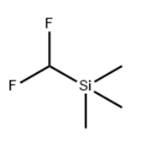
Description
A direct nucleophilic difluoromethylation reagent. The nucleophilic activation of the silicon center
with Lewis base initiators allows transfer of the difluoromethyl moiety to electrophiles such as
aldehydes, ketones, and aldimines. The copper-mediated difluoromethylation of halides using
TMSCF2H tolerates amine, ether, amide, ester, aromatic bromide, and protected alcohol
functionalities in aryl iodides and occurs in high yield and stereoselectivity with vinyl iodides.
Uses
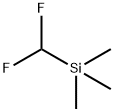
(Difluoromethyl)trimethylsilane, can be used for difluoromethylation of carbonyl compounds. Difluromethylated compounds are very useful in materials science and medicinal and agricultural chemistry.
Reactions
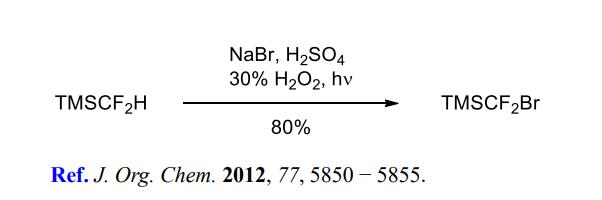
(1) Direct bromination to prepare TMSCF2Br.
(2) Difluoromethylation of aldehydes and ketones.
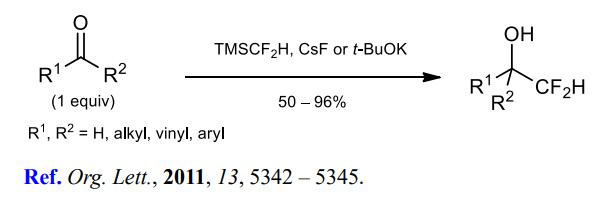
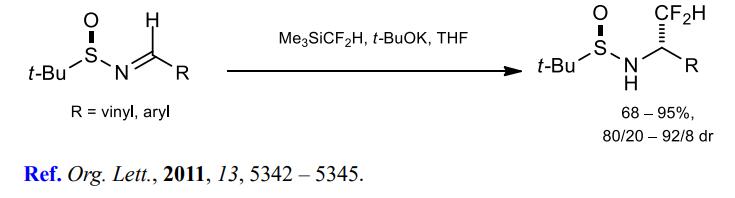
(3) Difluoromethylation of aldimines.
(4) Difluoromethylation of aryl and vinyl iodides.

References
[1] MIKHAIL D. KOSOBOKOV. Difluoro(trimethylsilyl)acetonitrile: Synthesis and Fluoroalkylation Reactions[J]. The Journal of Organic Chemistry, 2012, 77 13: 5850-5855. DOI:
10.1021/jo301094b.[2] YANCHUAN ZHAO. Efficient and Direct Nucleophilic Difluoromethylation of Carbonyl Compounds and Imines with Me3SiCF2H at Ambient or Low Temperature[J]. Organic Letters, 2011, 13 19: 5342-5345. DOI:
10.1021/ol202208b.[3] PATRICK S. FIER John F H. Copper-Mediated Difluoromethylation of Aryl and Vinyl Iodides[J]. Journal of the American Chemical Society, 2012, 134 12: 5524-5527. DOI:
10.1021/ja301013h.