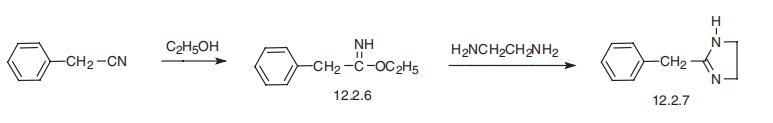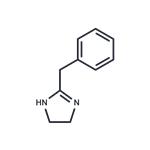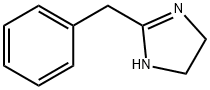
Tolazoline
- Product NameTolazoline
- CAS59-98-3
- CBNumberCB0236054
- MFC10H12N2
- MW160.22
- EINECS200-448-9
- MDL NumberMFCD00005182
- MOL File59-98-3.mol
Chemical Properties
| Melting point | 66-69 °C(lit.) |
| Boiling point | 188°C/20mmHg(lit.) |
| Density | 1.0849 (rough estimate) |
| refractive index | 1.6392 (estimate) |
| storage temp. | 2-8°C |
| solubility | alcohol: freely soluble |
| pka | pKa 10.3(H2O t undefined I = 0.1) (Uncertain) |
| form | Solid |
| color | Crystals from pet ether |
| Merck | 14,9506 |
| CAS DataBase Reference | 59-98-3(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | CHH9H12AQ3 |
| ATC code | C04AB02,M02AX02 |
| NIST Chemistry Reference | 1H-Imidazole, 4,5-dihydro-2-(phenylmethyl)-(59-98-3) |
| EPA Substance Registry System | 1H-Imidazole, 4,5-dihydro-2-(phenylmethyl)- (59-98-3) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Warning | |||||||||
| Hazard statements | H302+H312+H332-H315-H319-H335 | |||||||||
| Precautionary statements | P261-P280-P301+P312-P302+P352+P312-P304+P340+P312-P305+P351+P338 | |||||||||
| Hazard Codes | Xn | |||||||||
| Risk Statements | 20/21/22-36/37/38 | |||||||||
| Safety Statements | 26-28-36/37/39-45 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | NJ1925000 | |||||||||
| HS Code | 2933299090 | |||||||||
| Toxicity | cyt-ham:lng 62,500 mg/L GMCRDC 27,95,81 | |||||||||
| NFPA 704: |
|