Chemical Properties
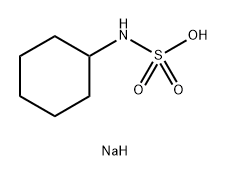
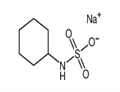
Cyclamate and its calcium or sodium salts are white crystalline powders with intensely sweet tastes. Cyclamate has a melting point of about 169–170 °C. It is soluble in water up to a concentration of about 1 g in 7.5 ml, whereas the calcium and sodium salts are slightly more soluble, to about 1 g in 4 ml of water. Cyclamate is rather acidic (the pH of a 10% aqueous solution being 0.8–1.6), whereas similar calcium and sodium salts solutions are neutral (pH 5.5–7.5). Cyclamate is relatively heat-stable, microbiologically inert, and non-hygroscopic.
Sweetener
Cyclamate is an odorless white crystalline powder used as a nonnutritive sweetener. The name usually denotes either calcium cyclamate or sodium cyclamate, which are salts of cyclohexylsulfamic acid (C6H11NHSO3H). These compounds are stable to heat and are readily soluble in water. Cyclamates taste very sweet, with about 30 times the sweetening power of sucrose. They are sweeteners in baked goods, confections, desserts, soft drinks, preserves, and salad dressings. They are often combined with saccharin to produce a synergistic sweetening effect.

Banned
After saccharin, cyclamate was the most commonly used sweetener until it was banned in the USA in 1970, after which many countries followed suit.