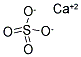Description
Phosphogypsum is a moist, fine powder with a free water content of ca. 20 – 30 %
and considerable amounts of impurities, the exact impurities and their amounts depending on
the rock and the specific process. The radioactive substances present in small amounts in sedimentary phosphate rock are partly transferred to
the phosphogypsum as 226Ra, leading to slightly
increased radioactivity of such gypsums.
About 1.7 t of gypsum is obtained per tonne of
raw phosphate, corresponding to 5 t of gypsum
per tonne of phosphorus pentoxide produced.
Uses
Anhydrous: insoluble anhydrite is used in cement formulations and as a paper filler. Soluble anhydride, because of its strong tendency to absorb moisture, is useful as a drying agent for solids, organic liquids and gases; the desiccant used in laboratory and industry is known under the name Drierite. This material can be regenerated repeatedly and reused without noticeable decrease in its desiccating efficiency. The hemihydrate is used for wall plasters; wallboard; tiles and blocks for the building industry; moldings; statuary; in the paper industry. The dihydrate is used in the manufacture of portland cement; in soil treatment to neutralize alkali carbonates and to prevent loss of volatile and dissolved nitrogenous compounds by volatilization and leaching; for the manufacture of plaster of Paris, artificial marble; as a white pigment, filler or glaze in paints, enamels, pharmaceuticals, paper, insecticide dusts, yeast manufacture, water treatment, polishing powders; in the manufacture of sulfuric acid, CaC2, (NH4)2SO4, porous polymers. Pharmaceutic aid (in plaster casts).
Definition
calcium sulphate: A white solidcompound, CaSO
4; r.d. 2.96; 1450°C.It occurs naturally as the mineralanhydrite, which has a rhombicstructure, transforming to a monoclinicform at 200°C. More commonly,it is found as the dihydrate,gypsum, CaSO
4.2H
2O (monoclinic;r.d. 2.32). When heated, gypsumloses water at 128°C to give thehemihydrate, 2CaSO
4.2H
2O, betterknown as plaster of Paris. Calciumsulphate is sparingly soluble in waterand is a cause of permanent hardnessof water. It is used in the manufacture of certain paints, ceramics,and paper. The naturally occurringforms are used in the manufacture ofsulphuric acid.
Definition
A powdered mixture of calcium
silicates and aluminates, which is made by
heating limestone (CaCO3) with clay, and
grinding the result. When mixed with
water, reactions occur with the water
(hence the name hydraulic cement) and a
hard solid aluminosilicate is formed.
General Description
White or nearly white, odorless, crystalline solid.
Reactivity Profile
CALCIUM SULFATE HEMIHYDRATE is generally of low reactivity. Can serve as an oxidizing agent under forcing conditions. Incompatible with aluminum (at high temperatures) and diazomethane.
Agricultural Uses
Calcium sulphate (CaSO
4) is a white solid that occurs
naturally as a mineral anhydrite. It is found more
commonly as dihydrate, called gypsum (CaSO
4·2H
2O).
When heated, gypsum loses water at 128°C to give a
hemihydrate (2CaSO
4·H
2O), also known as plaster of
Paris.
Calcium sulphate is sparingly soluble in water and
makes water permanently hard. CaSO
4 is used in the
manufacture of certain paints, ceramics and paper. Its
naturally occurring form is used in sulphuric acid
manufacture.
Gypsum is the cheapest and the most useful material
in reclamation of sodic soils.Calcium, solubilized from
gypsum, replaces sodium, leaving behind the watersoluble
sodium sulphate, which is leached out as a result
of the following reactions in the soil:
Since both reactions are reversible, adequate leaching
arrangements have to be made to remove sodium
sulphate.
The application of about 40 t/ha of gypsum in Nevada
(USA) was seen to increase water infiltration and the
depth of water penetration substantially. These two
measures increased hay yield up to 2.3 t/ha per year.
Gypsum requirement (GR) is the amount of gypsum
necessary to be added to reclaim soil and is calculated
using the formula:
The gypsum requirement is equivalent to (Na
x)×4.50
metric tons of gypsum per hectare for a 30 cm fixed
depth, where Na, is the milliequivalent of exchangeable
sodium to be replaced by calcium from the added gypsum.