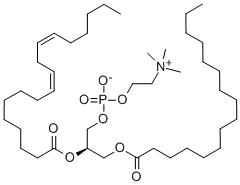
Description
Food-grade lecithin is obtained from soybeans and other plant
sources. It is a complex mixture of acetone-insoluble phosphatides
that consists chiefly of phosphatidyl choline, phosphatidyl etha nolamine, and phosphatidyl inositol, combined with various
amounts of other substances such as triglycerides, fatty acids, and
carbohydrates. Refined grades of lecithin may contain any of these
components in varying proportions and combinations depending
on the type of fractionation used. In its oil-free form, the prepon-derance of triglycerides and fatty acids is removed and the product
contains 90% or more of phosphatides representing all or certain
fractions of the total phosphatide complex. The consistency of
both natural grades and refined grades of lecithin may vary from
plastic to fluid, depending upon free fatty acid and oil content, and
upon the presence or absence of other diluents. Its color varies
from light yellow to brown, depending on the source, on crop
variations, and on whether it is bleached or unbleached. It is
odorless or has a characteristic, slight nutlike odor and a bland
taste. Edible diluents, such as cocoa butter and vegetable oils, often
replace soybean oil to improve functional and flavor characteris tics. Lecithin is only partially soluble in water, but it readily
hydrates to form emulsions. The oil-free phosphatides are soluble
in fatty acids, but are practically insoluble in fixed oils. When all
phosphatide fractions are present, lecithin is partially soluble in
alcohol and practically insoluble in acetone.
Chemical Properties
Lecithins vary greatly in their physical form, from viscous
semiliquids to powders, depending upon the free fatty acid content.
They may also vary in color from brown to light yellow, depending
upon whether they are bleached or unbleached or on the degree of
purity. When they are exposed to air, rapid oxidation occurs, also
resulting in a dark yellow or brown color.
Lecithins have practically no odor. Those derived from vegetable
sources have a bland or nutlike taste, similar to that of soybean oil.
Occurrence
Lecithin is found in foods such as eggs, beef liver, and peanuts. Commercial sources are available
Uses
Lecithin is an emulsifier that is a mixture of phosphatides which are
typically surface-active. it is now commercially obtained from soy-
beans; previously it was obtained from egg yolk. it is used in marga-
rine as an emulsifier and antispatter agent; in chocolate manufacture
it controls flow properties by reducing viscosity and reducing the
cocoa butter content from 3 to 5%; it is used as a wetting agent in
cocoa powder, fillings, and beverage powders; an antisticking agent
in griddling fat; and in baked goods to assist the shortening mix
with other dough ingredients and to stabilize air cells. typical usage
levels range from 0.1 to 1.0%.
Uses
egg lecithin is emollient and particularly recommended for sensitive skin.
Uses
lecithin is a natural emollient, emulsifier, anti-oxidant, and spreading agent, lecithin is a hydrophilic ingredient that attracts water and acts as a moisturizer. generally obtained for cosmetic products from eggs and soybeans, it is found in all living organisms.
Uses
Edible and digestible surfactant and emulsifier of natural origin. Used in margarine, chocolate and in the food industry in general. In pharmaceuticals and cosmetics. Many other industrial uses, e.g. treating leather and textiles.
Uses
lecithin (hydrogenated) is an emulsifier.
Production Methods
Lecithins are essential components of cell membranes and, in
principle, may be obtained from a wide variety of living matter. In
practice, however, lecithins are usually obtained from vegetable
products such as soybean, peanut, cottonseed, sunflower, rapeseed,
corn, or groundnut oils. Soybean lecithin is the most commercially
important vegetable lecithin. Lecithin obtained from eggs is also
commercially important and was the first lecithin to be discovered.
Vegetable lecithins are obtained as a by-product in the vegetable
oil refining process. Polar lipids are extracted with hexane and, after
removal of the solvent, a crude vegetable oil is obtained. Lecithin is
then removed from the crude oil by water extraction. Following
drying, the lecithin may be further purified.
With egg lecithin, a different manufacturing process must be
used since the lecithin in egg yolks is more tightly bound to proteins
than in vegetable sources. Egg lecithin is thus obtained by solvent
extraction from liquid egg yolks using acetone or from freeze-dried
egg yolks using ethanol (95%).
Synthetic lecithins may also be produced.
Definition
ChEBI: A glycerophosphocholine compound having O-acyl substituents at both the 1- and 2-positions of the glycerol. It is a major constituent of cell membranes.
Pharmaceutical Applications
Lecithins are used in a wide variety of pharmaceutical applications. They are also used in cosmetics and food products.
Lecithins are mainly used in pharmaceutical products as
dispersing, emulsifying, and stabilizing agents, and are included in
intramuscular and intravenous injections, parenteral nutrition
formulations, and topical products such as creams and ointments.
Lecithins are also used in suppository bases, to reduce the
brittleness of suppositories, and have been investigated for their
absorption-enhancing properties in an intranasal insulin formulation.
Lecithins are also commonly used as a component of enteral
and parenteral nutrition formulations.
There is evidence that phosphatidylcholine (a major component
of lecithin) is important as a nutritional supplement to fetal and
infant development. Furthermore, choline is a required component
of FDA-approved infant formulas. Other studies have indicated
that lecithin can protect against alcohol cirrhosis of the liver, lower
serum cholesterol levels, and improve mental and physical
performance.
Liposomes in which lecithin is included as a component of the
bilayer have been used to encapsulate drug substances; their
potential as novel delivery systems has been investigated. This
application generally requires purified lecithins combined in specific
proportions.
Therapeutically, lecithin and derivatives have been used as a
pulmonary surfactant in the treatment of neonatal respiratory
distress syndrome.
Biochem/physiol Actions
It also acts as a source of lipid messengers/ bioactive lipids including: lysophosphatidylcholine, diacylglycerol, phosphatidic acid, lysophosphatidylcholine, arachidonic acid and platelet activating factor. Phosphatidylcholine is produced in the liver by the CDP-choline (cytidine diphosphocholine) pathway.
Side effects
Lecithin may be safe when used as a supplement at a maximum dose of 30 grams per day for up to 6 weeks. It may have side effects, including diarrhoea, nausea, stomach pain or a feeling of fullness. When applied to the skin: Lecithin is probably safe for most adults. When used medicinally or as a drug carrier, no related serious adverse reactions have been reported.
Safety
Lecithin is a component of cell membranes and is therefore
consumed as a normal part of the diet. Although excessive
consumption may be harmful, it is highly biocompatible and oral
doses of up to 80 g daily have been used therapeutically in the
treatment of tardive dyskinesia. When used in topical formulations,
lecithin is generally regarded as a nonirritant and nonsensitizing
material. The Cosmetic Ingredients Review Expert Panel
(CIR) has reviewed lecithin and issued a tentative report revising the
safe concentration of the material from 1.95% to 15.0% in rinse-off
and leave-in products. They note, however, that there are
insufficient data to rule on products that are likely to be inhaled.
storage
Lecithins decompose at extreme pH. They are also hygroscopic and
subject to microbial degradation. When heated, lecithins oxidize,
darken, and decompose. Temperatures of 160–180°C will cause
degradation within 24 hours.
Fluid or waxy lecithin grades should be stored at room
temperature or above; temperatures below 10°C may cause
separation.
All lecithin grades should be stored in well-closed containers
protected from light and oxidation. Purified solid lecithins should
be stored in tightly closed containers at subfreezing temperatures.
Purification Methods
Lecithin from hen egg white is purified by solvent extraction and chromatography on alumina. It is suspended in H2O and kept frozen until required [Lee & Hunt J Am Chem Soc 106 7411 1984, Singleton et al. J Am Oil Chem Soc 42 53 1965]. For purification of commercial egg lecithin, see Pangborn [J Biol Chem 188 471 1951].
Incompatibilities
Incompatible with esterases owing to hydrolysis.
Regulatory Status
GRAS listed. Accepted for use as a food additive in Europe.
Included in the FDA Inactive Ingredients Database (inhalations; IM
and IV injections; otic preparations; oral capsules, suspensions and
tablets; rectal, topical, and vaginal preparations). Included in
nonparenteral and parenteral medicines licensed in the UK.
Included in the Canadian List of Acceptable Non-medicinal
Ingredients.