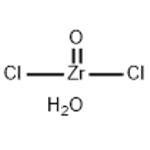
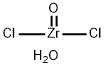Synthesis
Zircon is melted with caustic soda, rinsed, desiliconized, and then reacted with sulfuric acid, and then ammonia water is added to obtain zirconium hydroxide precipitate. Finished zirconium oxychloride.
Chemical Properties
Tetragonal prisms or needles. Deliquescent in moist air, evolves HCl and becomes dull in dry air. Soluble in H3O (slight hydrolysis) and alcohol. Lower hydrates are formed by heating a stream of HCl. It Liberates HCl on heating in air, and the solubility in water is gradually lost; it reverts to the oxide on calcination. Precipitation of an alcoholic solution with ether or acetone yields dizirconyl chloride Zr aO3Cl a -5HaO, which is sparingly soluble in water. The same compound deposits when a dilute aqueous solution of zirconyl chloride is allowed to stand for a month.
Chemical Properties
solid
Uses
Zirconium dichloride oxide is utilized as a precursor to prepare other zirconium compounds. It is employed in acid dyes, pigment toners and antiperspirants. It finds application as a rubber additive and a fiber treatment agent. It is also used in paint drying, refractories, ceramics and glaze.
Production Methods
Since zircon ZrSiO4, a mineral found in nature, is more difficult to work with, it is better to start from zirconia ZrO2 (baddeleyite), which is calcined, finely ground (the coarser particles are screened off with silk gauze) and converted to the sulfate by evaporation or treatment for several days with an excess of the warm cone. H2SO4. The solid residue, which consists of Zr(SO4)2 and unreacted ZrO2, is taken up in water (the solid is added in small portions to prevent solution heating). The sulfate dissolves slowly, and its solution may be aided by acidifying the water with some hydrochloric acid. The resultant milky suspension, which contains solid undissolved ZrO2 and SiO2 (or ZrSiO4), is allowed to stand for several hours and filtered. The weakly acidic sulfuric acid solution is precipitated with ammonia, and the hydroxide is filtered off. If the precipitate still exhibits a high Si content, it is dissolved in cone and hydrochloric acid and the solution is evaporated to dryness; this procedure is repeated several times. SiO2 and some basic zirconium chloride become the insoluble residue when redissolved in water. If no Si is evident in the hydroxide, the fresh gel is dissolved in cold hydrochloric acid, and the oxychloride is allowed to crystallize by adding cone, hydrochloric acid, or saturating it with HCl. The crystals are filtered and washed with 8N HCl.
General Description
This product is used as a potential green catalyst.
Purification Methods
Recrystallise the chloride several times from water [Ferragina et al. J Chem Soc, Dalton Trans 265 1986]. Recrystallisation from 8M HCl gives the octahydrate as white needles on concentrating. It is also formed by hydrolysing ZrCl4 with water. After one recrystallisation from H2O, 99+% grade zirconyl chloride had Ag, Al, As, Cd, Cu, Hf, Mg, Na, Sc and V at 20, 1.8, 0.6, 0.6, 0.4, 8.4, 0.4, 2.4, 80 and 3 ppm, respectively. (See above.) METAL-ORGANIC COMPOUNDS This section contains metal-organic compounds as well as ammonium and metal salts of organic acids. (For Introduction see p 445.)