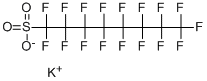
Chemical Properties
Brown solid
Uses
Potassium heptadecafluoro-1-octanesulfonate is a persistent environmental pollutant that may cause adverse health effects in humans.
Uses
Perfluorinated surfactants consist of a broad class of fluorinated
chemicals of differing structures, physical–chemical
properties, and modes of toxic action. This class of
compounds is made up of fully fluorinated organic
compounds that due to their unique chemical properties can
be used in a variety of industrial processes and products. Some
characteristics of these chemicals are the ability to repel water
and oil, reduce surface tension, catalyze oligomerization and
polymerization, and maintain their properties under extreme
conditions. Sulfonyl-based perfluorochemicals are produced
by an electrochemical fluorination (ECF) process where
hydrogen atoms of a feedstock are replaced with fluorine
atoms. Perfluorooctane sulfonyl fluoride (POSF) is the principal
product from this process and it has been used in
a variety of products that can be divided into three main
categories: surface treatments (carpet and textile protection),
paper protection (grease, oil, and water resistance), and
performance chemicals (firefighting foams, mining surfactants,
and electronic etching baths). Depending on the degree of
derivatization and polymerization, POSF products can
degrade to perfluorooctane sulfonate (PFOS), a stable end
product that is resistant to degradation. Commercially, ECFproduced
PFOS consists of linear (70%) and branched (30%) isomers that have differing environmental and toxicological
properties. However, due to the presence of PFOS in biota
from sites remote from production, as well as its presence in
human blood, PFOS has been voluntarily withdrawn from
commercial production in the United States. In Europe, the use
of PFOS is regulated by Registration, Evaluation, Authorisation
and Restriction of Chemical (REACH) under Annex XVII.
Environmental Fate
As a result of the production and use of PFOS and its precursors,
it has been released into the environment through variety
of waste streams. The environmental partitioning behavior of
PFCs is unusual and differs from many other persistent organic
pollutants in that PFOS-based substances are both oleophobic
and hydrophobic. As a result, an octanol/water partitioning
coefficient (Kow) for PFOS has not been determined. PFOS is
persistent in the environment and does not hydrolyze, undergo
direct or indirect photolysis, or biodegrade to any significant
degree. While PFOS has low volatility, several PFOS precursors
are considered volatile, including EtFOSE and N-methyl
perfluorooctane sulfonamidoethanol alcohols, which may
account for its global distribution. As a result of its persistence
and widespread distribution, PFOS was recently added to the
Stockholm Convention’s list of persistent organic pollutants. If
released into soil, sediment, or sludge, PFOS is expected to
adsorb strongly to organic and inorganic components. Due to
these properties, PFOS is expected to persist in soils, sediments,
and sludge. If released into water, PFOS is expected to remain
in the water compartment unless it is assimilated into organisms
or adsorbed onto particulate matter and potentially
deposited into sediments. Volatilization from water surfaces or
biodegradation is not expected to be important fate processes.
PFOS has the potential to bioaccumulate in aquatic organisms.
Laboratory-based bioconcentration factors for PFOS range
from 56 to over 1000 while field-based bioaccumulation
factors range from 830 to 125 000. The field-based bioaccumulation
factors for PFOS may be overestimated due to
metabolism of accumulated perfluorinated derivatives of
PFOS. Trophic magnification of PFOS in food webs has also
been studied in several different aquatic systems. In a bottlenose
dolphin food web, the trophic magnification factor
(TMF) based on whole body concentrations ranged between
1.8 and 6.3. In an Eastern Arctic marine food web with glaucous
gulls as the top predator, the TMF for PFOS was 3.1 while in a Western Canadian Arctic food web the TMF was 1.9.
Finally, in a Lake Ontario food web, the TMF for PFOS was 3.8.
In a terrestrial environment that examined a food chain that
included lichen–caribou–wolf, the TMF for PFOS ranged from
2.3 to 2.6. Overall these results indicate that PFOS is magnified
up through different trophic levels in both aquatic and terrestrial
food webs.
Toxicity evaluation
The mechanisms governing the toxicity of PFOS to biological
systems are still under investigation. Potential modes of action
that have been identified include competition with fatty acids
for carrier protein sites, cholesterol synthesis, and bioenergetics.
Other studies suggest that PFOS may alter peroxisomal
fatty acid b-oxidation.