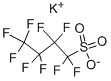
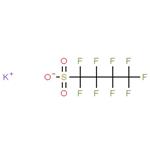
Description
Potassium nonafluoro-1-butanesulfonate is a chemical compound that is used to remove trifluoroacetic acid from wastewater. It can also be used as an analytical reagent to measure cytosolic calcium concentrations in cells. In addition, it has been shown to have a high degree of chemical stability, but it may react with hydrogen fluoride and cause toxicity in humans.
Safety
Potassium nonafluoro-1-butanesulfonate can cause skin irritation, severe eye damage, and serious eye irritation. Additionally, it may cause respiratory irritation.
Chemical Properties
White crystal
Uses
Potassium nonafluoro-1-butanesulfonate (KFBS), a potassium salt of perfluorobutyl sulfonic acid, is a white crystalline solid with high thermal stability, low vapor pressure, and good solubility in polar solvents. It could be used to synthesize ionic liquids in cooperation with pyrrolidinium salts. These ionic liquids modify the surface of the cathode materials in lithium batteries with enhanced stability and performance. This multifunctional surfactant could be introduced into the SnO2 ETLs. The nonafluorocarbon alkyl chains of KFBS molecule bond with undercoordinated Sn2+ ions in SnO2, which effectively inhibits the charge recombination caused by defects related to oxygen vacancies; the potassium ion (K+) could ameliorate the crystal quality and increase the size of grains of perovskite, adjust the energy level matching between SnO2 and perovskite; the sulfonate group could interact with uncoordinated Pb2+ ions to reduce the surface defects and suppress carrier nonradiative recombination. The power conversion efficiencies increase from 20.6% for pristine devices to 23.21% for KFBS-modified devices[1].
Uses
Potassium Perfluoro-1-butanesulfonate is standard for environmental testing and research. Also used on studies to compare the effect of glimepiride and nateglinde in all patients with type-2 diabetes.
Synthesis
Potassium nonafluoro-1-butanesulfonate is prepared by the reaction of Nonafluorobutanesulfonyl fluoride and potassium hydroxide.
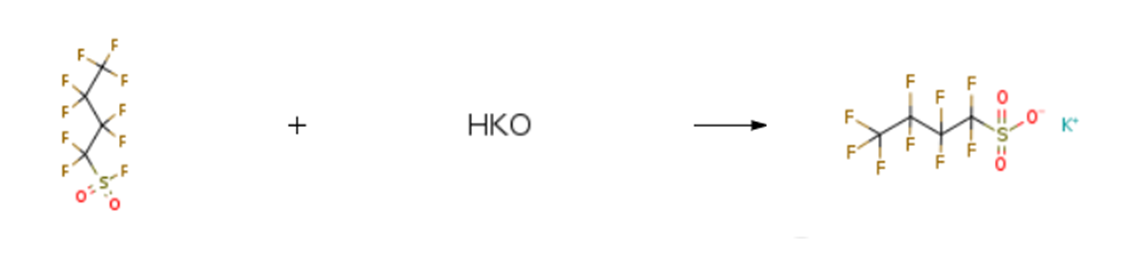
Purification Methods
Wash it with H2O and dry it in vacuo. When the K salt is distilled with 100% H2SO4, it gives the free acid which can be distilled (b 105o/22mm, 210-212o/760mm) and then converted to the pure K salt. [Gramstad & Haszeldine J Chem Soc 2640 1957, Beilstein 2 IV 818.]
References
[1] Zhaohui Wu. “Multifunctional molecule of potassium nonafluoro-1-butanesulfonate for high-efficient perovskite solar cells.” Chemical Engineering Journal 449 (2022): Article 137851.