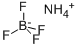Chemical Properties
white crystalline powder
Uses
Ammonium fluoroborate is used as an analytical reagent. It is also applied in the textile printing and dyeing industry and used as a catalyst for resin finishing. It is mainly used for the high temperature fluxing action required by the metals industry; catalyst; in flame retardants and acts as a solid lubricant in cutting-oil emulsions for aluminum rolling and forming.
General Description
Odorless white crystals. Sinks and mixes with water.
Air & Water Reactions
Sublimes above 238°C. to form toxic fumes [AAR 1991]. Water soluble.
Reactivity Profile
Acidic inorganic salts are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Health Hazard
INHALATION: May cause irritation of respiratory passages, nose bleeds, and nausea. EYES: May irritate.
Fire Hazard
Behavior in Fire: Sublimes above 238°C yielding toxic fumes.
Safety Profile
A poison and strong irritant. See also FLUORIDES and BORON COMPOUNDS. When heated to decomposition it emits very toxic fumes of F-, NO,, and NH3.
Purification Methods
Crystallise it from conductivity water (1mL/g) between 100o and 0o. 450 Purification of Inorganic Compounds