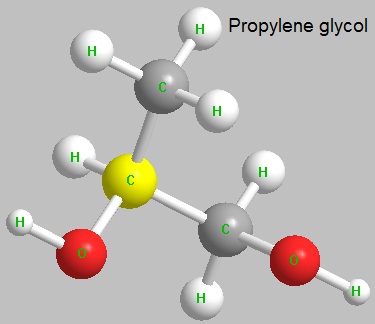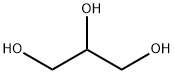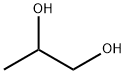
Propylene Glycol Usp
- Product NamePropylene Glycol Usp
- CAS57-55-6
- CBNumberCB00125222
- MFC3H8O2
- MW76.09
- EINECS200-338-0
- MDL NumberMFCD00064272
- MOL File57-55-6.mol
Chemical Properties
| Melting point | -60 °C (lit.) |
| Boiling point | 187 °C (lit.) |
| Density | 1.036 g/mL at 25 °C (lit.) |
| vapor density | 2.62 (vs air) |
| vapor pressure | 0.08 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2940 | PROPYLENE GLYCOL |
| Flash point | 225 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| pka | 14.49±0.20(Predicted) |
| form | Viscous Liquid |
| color | APHA: ≤10 |
| Specific Gravity | 1.038 (20/20℃)1.036~1.040 |
| PH | 6-8 (100g/l, H2O, 20℃) |
| Odor | at 100.00 %. odorless very slight alcoholic |
| Odor Type | odorless |
| biological source | synthetic |
Safety
| Symbol(GHS) |

|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H370-H372 | |||||||||
| Precautionary statements | P501-P260-P270-P264-P308+P311-P405 | |||||||||
| Safety Statements | 24/25 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | TY2000000 | |||||||||
| Autoignition Temperature | 779 °F | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29053200 | |||||||||
| Hazardous Substances Data | 57-55-6(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 orally in Rabbit: 19400 - 36000 mg/kg LD50 dermal Rabbit 20800 mg/kg | |||||||||
| NFPA 704: |
|