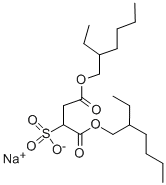
Chemical Properties
Docusate sodium is a white or almost white, waxlike, bitter tasting,
plastic solid with a characteristic octanol-like odor. It is hygroscopic
and usually available in the form of pellets, flakes, or rolls of tissuethin
material.
Chemical Properties
white solid, often supplied as an aqueous solution
Uses
Sodium salt of Docusate, used for the treatment of constipation, acting as a laxative or stool softener. Also used in the synthesis of electrospun fibres for tailored and controlled antibiotic drug release.
Uses
dioctyl sodium sulfosuccinate is a mild surfactant used as a cleans ing agent.
Uses
Forms reverse micelles in hydrocarbon solvents; Suitable for the solubilization of the major myelin transmembrane proteolipid
Uses
anticholinergic, treatment of motion sickness
Uses
Dioctyl sulfosuccinate sodium salt is a wetting and emulsifying agent that is slowly soluble in water, having a solubility of 1 g in 70 ml of water. It functions as a wetting agent in fumaric acid-containing powdered fruit drinks to help the acid dissolve in water. It is used as a stabilizing agent on gums at not more than 0.5% by weight of the gum. It is used as a flavor potentiator in canned milk where it improves and maintains the flavor of the sterilized milk during storage. It also functions as a processing aid in the manufacture of unrefined sugar. It is also termed sodium dioctylsulfosuccinate.
Definition
ChEBI: Sodium docusate is an organic sodium salt.
Production Methods
Maleic anhydride is treated with 2-ethylhexanol to produce dioctyl
maleate, which is then reacted with sodium bisulfite.
brand name
Colace (Roberts Pharmaceutical); Correctol Stool Softener Laxative (Schering-Plough HealthCare); Dialose (Johnson & Johnson-Merck Consumer); Doxinate (Hoechst-Roussel); D-S-S (Parke-Davis); Modane Soft (Savage); Molofac (Bristol-Myers Squibb).
General Description
Odorless colorless to white waxy solid. Sinks and mixes slowly with water.
Air & Water Reactions
Mixes slowly with water.
Reactivity Profile
Docusate sodium causes foaming and spreading of water. Assists in putting out fires by water. [USCG, 1999].
Health Hazard
Liquid is strong irritant to eye and may irritate skin by removing natural oils. Ingestion causes diarrhea and intestinal bloating.
Fire Hazard
Behavior in Fire: Causes foaming and spreading of water. Assists in putting out fires by water.
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Docusate sodium and docusate salts are widely used as anionic
surfactants in pharmaceutical formulations. Docusate sodium is
mainly used in capsule and direct-compression tablet formulations
to assist in wetting and dissolution.
Safety Profile
Poison by intravenous
route. Moderately toxic by ingestion and
intraperitoneal routes. A skin and severe eye
irritant. See also ESTERS. When heated to
decomposition it emits toxic fumes of SOx
and Na2O.
Safety
Docusate salts are used in oral formulations as therapeutic agents
for their fecal softening and laxative properties. As a laxative in
adults, up to 500mg of docusate sodium is administered daily in
divided doses; in children over 6 months old, up to 75 mg in divided
doses is used. The quantity of docusate sodium used as an excipient
in oral formulations should therefore be controlled to avoid
unintended laxative effects. Adverse effects associated with
docusate sodium include diarrhea, nausea, vomiting, abdominal
cramps, and skin rashes. As with the chronic use of laxatives, the
excessive use of docusate sodium may produce hypomagnesemia.
Docusate salts are absorbed from the gastrointestinal tract and
excreted in bile; they may cause alteration of the gastrointestinal
epithelium. The gastrointestinal or hepatic absorption of other
drugs may also be affected by docusate salts, enhancing activity and
possibly toxicity. Docusate sodium should not be administered with
mineral oil as it may increase the absorption of the oil.
LD50 (mouse, IV): 0.06 g/kg
LD50 (mouse, oral): 2.64 g/kg
LD50 (rat, IP): 0.59 g/kg
LD50 (rat, oral): 1.9 g/kg
Solubility in organics
Dioctyl sodium sulfosuccinate (DSS) is the dioctyl ester of sodium sulfosuccinate (bis-2-ethyl-hexyl sodium sulfosuccinate). It dissolves slowly in water; at 25°C to the extent of 1.5 gm/100cc; at 70°C, 5.5 gm/100cc. It dissolves in oils, hydrocarbons, fats and waxs by heating above 75°C and remains in solution when cooled to room temperature. At room temperature, it is readily soluble in most organic solvents, both polar and non-polar. soluble in carbon tetrachloride, petroleum ether, naphtha, xylene, dibutyl phthalate, liquid petroleum, acetone, alcohol, vegetable oils.
storage
Docusate sodium is stable in the solid state when stored at room
temperature. Dilute aqueous solutions of docusate sodium between
pH 1–10 are stable at room temperature. However, at very low pH
(<1) and very high pH (>10) docusate sodium solutions are subject
to hydrolysis.
The solid material is hygroscopic and should be stored in an airtight container in a cool, dry place.
Purification Methods
Dissolve it in MeOH and the inorganic salts which precipitate are filtered off. Water is added and the solution is extracted several times with hexane. The residue is evaporated to one-fifth its original volume, *benzene is added and azeotropic distillation is continued until no water remains. The solvent is evaporated. The white residual solid is crushed and dried in vacuo over P2O5 for 48hours [El Seoud & Fendler J Chem Soc, Faraday Trans 1 71 452 1975]. [Beilstein 4 IV 114.] It solubilises major myelin trans membrane proteolipids, and forms reverse micelles in hydrocarbon solvents.
Incompatibilities
Electrolytes, e.g. 3% sodium chloride, added to aqueous solutions
of docusate sodium can cause turbidity. However, docusate
sodium possesses greater tolerance to calcium, magnesium, and
other polyvalent ions than do some other surfactants. Docusate
sodium is incompatible with acids at pH < 1 and with alkalis at pH
> 10.
Regulatory Status
GRAS listed. Included in the FDA Inactive Ingredients Database
(IM injections; oral capsules, suspensions, and tablets; also topical
formulations). Included in nonparenteral medicines licensed in the
UK. Included in the Canadian List of Acceptable Non-medicinal
Ingredients.