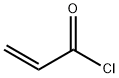
Chemical Properties
Acryloyl chloride is a highly flammable, polymerizable, and toxic (inhalation), light yellow liquid. A lacrimator.
Uses
Acryloyl chloride is used in the production of plastics. It plays an important role in the preparation of acrylate monomers and polymers. It also acts as a substrate for cross-metathesis. Further, it is utilized in organic synthesis for the introduction of acrylic groups into other compounds.
Synthesis Reference(s)
Journal of the American Chemical Society, 72, p. 72, 1950
DOI: 10.1021/ja01157a020
General Description
A liquid. Boiling point 75°C. Used to make plastics. Polymerization in a closed container can cause pressurization and explosive rupture.
Air & Water Reactions
Polymerizes readily upon exposure to oxygen in the air. Reacts exothermically with water to give hydrochloric acid and acrylic acid.
Reactivity Profile
Acrylyl chloride is incompatible with strong oxidizing agents, alcohols, amines, alkali. Polymerizes readily upon exposure to oxygen. May react vigorously or explosively if mixed with diisopropyl ether or other ethers in the presence of trace amounts of metal salts [J. Haz. Mat., 1981, 4, 291].
Fire Hazard
When heated to decomposition, Acrylyl chloride emits toxic fumes of chlorides. Decomposes in water.
Potential Exposure
May be used as a monomer in preparation of specialty polymers or as a chemical intermediate
Shipping
UN3383 Poisonous Toxic by inhalation liquid, flammable, n.o.s. with an LC50 # 200 mL/m3 and saturated vapor concentration ≥500 LC50, Hazard class: 6.1; Labels: 6.1-Poisonous materials, 3-Flammable liquid, Technical Name Required, Inhalation Hazard Zone A. UN2924 Flammable liquids, corrosive, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material, Technical Name Required
Incompatibilities
Use MEHQ (monomethyl ether of hydroquinone) as an inhibitor. Incompatible with oxidizers, polymerizes on contact with oxygen; alcohols, amines, alkalis. Reacts violently with water, releasing hydrochloric acid and acrylic acid. Attacks some metals.
Toxics Screening Level
The initial threshold screening level (ITSL) for acryloyl chloride is 0.3 μg/m3 based on an
annual averaging time.