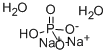Chemical Properties
Anhydrous Dibasic sodium phosphate occurs as a white powder. The dihydrate occurs as white or almost white, odorless crystals.
The heptahydrate occurs as colorless crystals or as a white granular or caked salt that effloresces in warm, dry air. The dodecahydrate occurs as strongly efflorescent, colorless or transparent crystals.
Uses
Dibasic sodium phosphate is used in conjunction with trisodium phosphate in foods and water softening treatment. In foods, it is used to adjust pH. Its presence prevents coagulation in the preparation of condensed milk. Similarly, it is used as an anti-caking additive in powdered products.
Preparation
Dibasic sodium phosphate can be generated by neutralization of phosphoric acid with sodium hydroxide:
H3PO4 + 2 NaOH → Na2HPO4 + 2 H2O
Biochem/physiol Actions
Dibasic Sodium Phosphate dehydrate is an important component of running buffer of denaturing gel electrophoresis.
Side effects
Gastrointestinal disturbances including diarrhea, nausea, and vomiting may occur following the use of dibasic sodium phosphate as an excipient in oral formulations. However, the level of dibasic sodium phosphate used as an excipient in a pharmaceutical formulation is not usually associated with adverse effects.
Metabolism
Approximately two-thirds of ingested phosphate is absorbed from the gastrointestinal tract, virtually all of it being excreted in the urine, and the remainder is excreted in the feces.