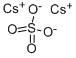Description
Cesium sulfate is used for equilibrium density gradient centrifugation, one of the most useful tools for fractionation and characterization of macromolecules, such as DNA. Cesium sulfate can be used as catalyst, e.g. catalyst of cesium sulfate/vanadium pentoxide is used in abiabatic multibed reactors to lower inlet temperature and catalyst cesium sulfate/zeolite is used in a pilot plant scale process to synthesize 4-methyl thiazol.
References
[1] Waclaw Szybalski, Use of cesium sulfate for equilibrium density gradient centrifugation, Methods in Enzymology, 1968, vol. 12, 330-360.
[2] Dmitry Yu. Murzin, Chemical Reaction Technology, 2015.
[3] James A. Schwarz, Cristian I. Contescu and Karol Putyera, Dekker Encyclopedia of Nanoscience and Nanotechnology, Volume 1, 2004.
[4] D W Kufe , P P Major , D Munroe , M Egan , D Herrick, Relationship between incorporation of 9-beta-D-arabinofuranosyladenine in L 1210 DNA and cytotoxicity, 1983.
[5] Le, F.K., Schneeweiss, P., Rauschenbeutel, A., Dynamical polarizability of atoms in arbitrary light fields: general theory and application to cesium. Eur. Phys. J. D. 2013, 67, 92.
[6] Xiufeng Zhang, Zhifeng Qin, Tahani Aldahri, Sohrab Rohani, Shan Ren, Weizao Liu, Separation and recovery of cesium sulfate from the leach solution obtained in the sulfuric acid baking process of lepidolite concentrate, 2021, 105537.
Chemical Properties
white or colourless odourless crystals
Uses
Cesium sulfate is the basic raw material for the preparation of various cesium salts, and can be used in trace analysis of aluminum and berkelium analysis reagent. It is often used as a liquid medium for density gradient centrifugation to separate and extract nucleic acids, suborganelles and plasmids
[4].
Uses
Cesium sulfate is used to prepare dense aqueous solutions for use in isopycnic centrifugation. It is also used for crystal growing purposes.
Uses
With V or V2O5 as catalyst for SO2 oxidation.
Preparation
Lepidolite is an important mineral resource for the preparation of lithium, rubidium and cesium
[5]. And cesium can be collected from the leaching solution by the process of crystallization, roasting, dissolution and solvent extraction. After the leaching solution is cooled from 85 °C to 35 °C. The mixture is calcined at 900 °C after most of Cs
+ is precipitated as CsAl(SO
4)
2.XH
2O, and the calcined product is dissolved in water to obtain Cs
+ mixed sulfuric acid solution
[6]. After four-stage countercurrent extraction, obtain the mixed sulfuric acid solutionis. Cs
+ (99.20%) was extracted and purified by 4-tert-butyl-2-(α-methylbenzyl)
phenol (t-BAMBP). The organic phase dissolved with CS
+ was scrubbing and stripped with sulfuric acid, and cesium sulfate products were obtained after crystallization.
Flammability and Explosibility
Non flammable
Purification Methods
Crystallise it from water (0.5mL/g) by adding ethanol and cooling.