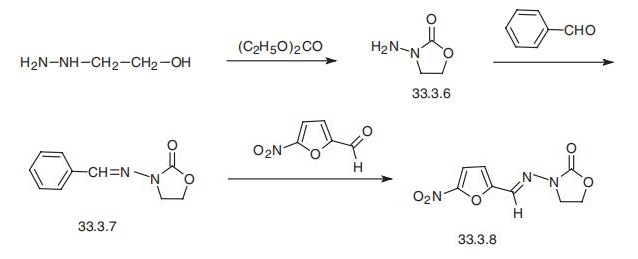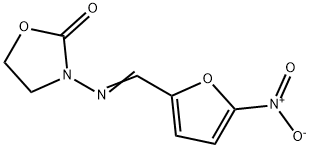
Furazolidon
Bezeichnung:Furazolidon
CAS-Nr67-45-8
Englisch Name:Furazolidone
CBNumberCB8452405
SummenformelC8H7N3O5
Molgewicht225.16
MOL-Datei67-45-8.mol
Synonyma
Furazolidon
Furazolidon physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 254-256°C (dec.) |
| Siedepunkt | 366.66°C (rough estimate) |
| Dichte | 1.5406 (rough estimate) |
| Brechungsindex | 1.7180 (estimate) |
| Flammpunkt | 2 °C |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| Löslichkeit | formic acid: soluble50mg/mL |
| Aggregatzustand | powder |
| pka | -1.98±0.20(Predicted) |
| Farbe | yellow |
| Biologische Quelle | synthetic |
| Sensitive | Light Sensitive |
| maximale Wellenlänge (λmax) | 365nm(DMSO)(lit.) |
| Merck | 14,4300 |
| BRN | 8317414 |
| Stabilität | Stable. Combustible. Incompatible with strong oxidizing agents. |
| InChIKey | PLHJDBGFXBMTGZ-UITAMQMPSA-N |
| IARC | 3 (Vol. 31, Sup 7) 1987 |
| Kennzeichnung gefährlicher | Xn,F |
| R-Sätze: | 62-40-36-20/21/22-11-68 |
| S-Sätze: | 36-22-36/37-16 |
| WGK Germany | 3 |
| RTECS-Nr. | RQ3675000 |
| TSCA | Yes |
| HS Code | 29349990 |
| Giftige Stoffe Daten | 67-45-8(Hazardous Substances Data) |