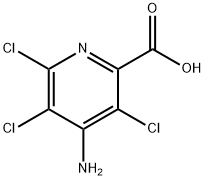
4-Amino-3,5,6-trichlorpyridin-2-carbonsure
Bezeichnung:4-Amino-3,5,6-trichlorpyridin-2-carbonsure
CAS-Nr1918-02-1
Englisch Name:Picloram
CBNumberCB8143725
SummenformelC6H3Cl3N2O2
Molgewicht241.46
MOL-Datei1918-02-1.mol
Synonyma
4-Amino-3,5,6-trichlorpyridin-2-carbonsure
4-Amino-3,5,6-trichlorpyridin-2-carbons?ure
4-Amino-3,5,6-trichlorpicolins?ure
4-Amino-3,5,6-trichlorpyridin-2-carbonsure physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 200 °C (dec.)(lit.) |
| Siedepunkt | 421℃ |
| Dichte | 1.9163 (rough estimate) |
| Brechungsindex | 1.6770 (estimate) |
| Flammpunkt | >110°(230°F) |
| storage temp. | 0-6°C |
| Löslichkeit | Soluble in acetone |
| Aggregatzustand | Granules |
| pka | 4.1(at 25℃) |
| Farbe | White, tan |
| Wasserlöslichkeit | 420 mg/L |
| maximale Wellenlänge (λmax) | 252nm(Phosphate buffer sol.)(lit.) |
| Merck | 14,7397 |
| BRN | 479075 |
| Expositionsgrenzwerte | OSHA PEL: 15 mg/m3 (total), 5 mg/m3 (respirable fraction); ACGIH TLV: TWA 10 mg/m3, STEL 20 mg/m3. |
| InChIKey | NQQVFXUMIDALNH-UHFFFAOYSA-N |
| LogP | 0.300 |
| CAS Datenbank | 1918-02-1(CAS DataBase Reference) |
| Kennzeichnung gefährlicher | Xi |
| R-Sätze: | 36 |
| S-Sätze: | 26 |
| RIDADR | 3077 |
| WGK Germany | 2 |
| RTECS-Nr. | TJ7525000 |
| TSCA | Yes |
| HazardClass | 9 |
| PackingGroup | III |
| HS Code | 2933399990 |
| Giftige Stoffe Daten | 1918-02-1(Hazardous Substances Data) |
| Toxizität | LD50 in rats, mice, rabbits, guinea pig, chickens, sheep, cattle (mg/kg): 8200, 2000-4000, 2000, 3000, 6000, >1000, >750 orally (Mullison) |



