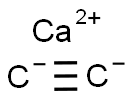
Calciumacetylid
Bezeichnung:Calciumacetylid
CAS-Nr75-20-7
Englisch Name:Calcium carbide
CBNumberCB5854217
SummenformelC2Ca
Molgewicht64.1
MOL-Datei75-20-7.mol
Synonyma
Calciumacetylid
Acetylenogen
Calciumacetylid physikalisch-chemischer Eigenschaften
| Schmelzpunkt | 447°C |
| Siedepunkt | 2300°C |
| Dichte | 2.22 g/mL at 25 °C(lit.) |
| storage temp. | water-free area |
| Löslichkeit | reacts with H2O |
| Aggregatzustand | pieces |
| Wichte | 2.22 |
| Farbe | Gray-black |
| Wasserlöslichkeit | hydrolyses |
| Sensitive | Moisture Sensitive |
| Merck | 14,1656 |
| BRN | 3909011 |
| Dielectric constant | 5.8 - 7.0(0.0℃) |
| Expositionsgrenzwerte | ACGIH: TWA 2 mg/m3 OSHA: TWA 5 mg/m3 NIOSH: IDLH 25 mg/m3; TWA 2 mg/m3 |
| Stabilität | Stability Reacts violently with water liberating highly flammable gas (acetylene). Do not use water if this material is involved in a fire. Incompatible with moisture, water, strong oxidizing agents, alcohols, hydrogen chloride, magnesium. |
| InChIKey | UIXRSLJINYRGFQ-UHFFFAOYSA-N |
| EPA chemische Informationen | Calcium carbide (75-20-7) |
| Kennzeichnung gefährlicher | F |
| R-Sätze: | 15-41-37/38 |
| S-Sätze: | 8-43-43A-39-26 |
| RIDADR | UN 1402 4.3/PG 2 |
| WGK Germany | 1 |
| F | 10-21 |
| TSCA | Yes |
| HazardClass | 4.3 |
| PackingGroup | II |
| HS Code | 28491000 |
| Giftige Stoffe Daten | 75-20-7(Hazardous Substances Data) |



