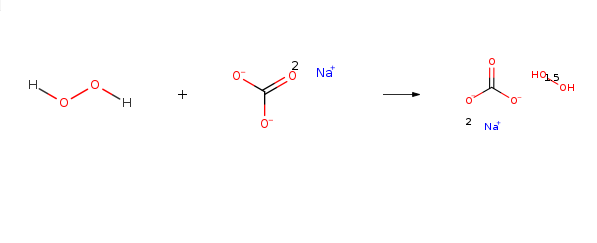
Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3)
Bezeichnung:Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3)
CAS-Nr15630-89-4
Englisch Name:Sodium percarbonate
CBNumberCB4853147
SummenformelCH5NaO5
Molgewicht120.04
MOL-Datei15630-89-4.mol
Synonyma
Dinatriumcarbonat, Verbindung mit Hydrogenperoxid(2:3)
Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3)
Dinatriumcarbonat, Verbindung mitHydrogenperoxid (2:3) physikalisch-chemischer Eigenschaften
| Dichte | 2.09[at 20℃] |
| Dampfdruck | < 10-3 Pa at 25°C |
| Aggregatzustand | Granular Powder |
| Farbe | White |
| Geruch (Odor) | Odorless |
| PH | About 10.5 at 1% concentration (20°C) |
| Wasserlöslichkeit | Soluble in water. |
| Sensitive | Moisture Sensitive |
| Decomposition | > 131 °F (> 55 °C) |
| LogP | -0.809 (est) |
| CAS Datenbank | 15630-89-4(CAS DataBase Reference) |
| EPA chemische Informationen | Sodium percarbonate (15630-89-4) |
| Kennzeichnung gefährlicher | O,Xn |
| R-Sätze: | 8-22-41-36/38 |
| S-Sätze: | 17-26-39-37/39 |
| RIDADR | UN 1479 5.1/PG 3 |
| WGK Germany | 1 |
| RTECS-Nr. | FG0750000 |
| F | 21 |
| TSCA | Yes |
| HazardClass | 5.1 |
| PackingGroup | II |
| HS Code | 28369990 |
| Giftige Stoffe Daten | 15630-89-4(Hazardous Substances Data) |
| Toxizität | mouse,LD50,oral,2200mg/kg (2200mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)LUNGS, THORAX, OR RESPIRATION: DYSPNEA,Toksikologicheskii Vestnik. Vol. (3), Pg. 46, 1994. |