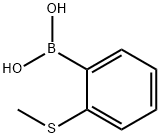
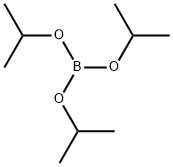
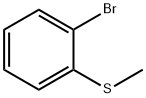
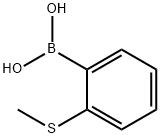
Part A: Preparation of 2-methylthiophenylboronic acid. 2-Bromothioanisole (29.0 g, 143 mmol) was dissolved in anhydrous tetrahydrofuran (400 mL) and the solution was cooled to -75°C. n-Butyllithium (62.0 mL, 2.5 M hexane solution, 155 mmol) was added slowly over 50 minutes. After stirring for 25 min, triisopropyl borate (46 mL, 199 mmol) was added dropwise over 35 min. The cold bath was removed and the reaction mixture was stirred at room temperature for 16 hours. The resulting solution was cooled in an ice bath and 6 M hydrochloric acid (100 mL) was added. The mixture was stirred at room temperature for 5 hours and then concentrated to half the original volume. The concentrate was partitioned between ether and water. The organic layer was extracted with 2 M sodium hydroxide solution, subsequently acidified with 6 M hydrochloric acid, concentrated again, and extracted several times with ether. The ether extracts were combined, dried with sodium sulfate, filtered and the solvent evaporated to give a beige solid product (20.4 g, 85% yield).1H-NMR (CDCl3) δ: 8.01 (dd, 1H, J = 7.3, J' = 1.4), 7.53 (dd, 1H, J = 7.7, J' = 1.1), 7.43 (td, 1H, J = 7.3, J' = 1.8), 7.34 (td, 1H, J = 7.3, J'= 1.5), 6.22 (s, 2H), 2.50 (s, 3H).