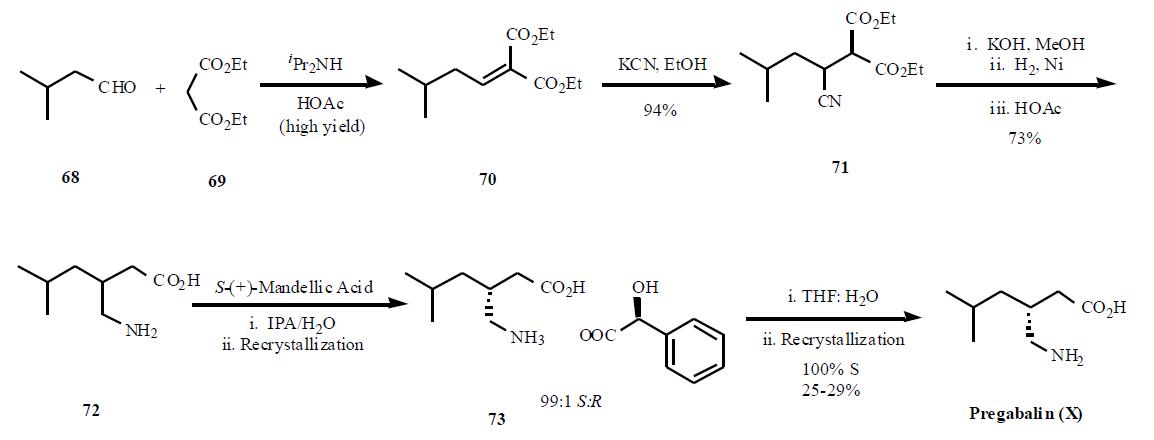
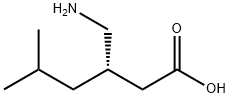
Several syntheses of pregabalin (X) have been disclosed in the literature, including process scale-up comparison of
several different routes. The most cost efficient route
as described in the publication is shown in the Scheme.
Condensation of diethyl malonate 69 in the presense of
diisopropyl amine in acetic acid gave a,b-unsaturated diester
70 in high yield. Reaction of the enone diester with
potassium cyanide gave cyano diester 71 in 95% yield. In a
remarkable three step, one pot process, the nitrile in 71 was
hydrolyzed followed by decarboxylation of one of the esters
to provide 72 in 73% yield. Resolution of the two
enantiomers was achieved using (S)-(+)-mandellic acid, one
of the best acid found after many salt screening, to give, after
two recrystallization, a 99:1 ratio of the desired diastereomer.Removal of the acid was done with wet THF instead of base
separation, to avoid salt impurities, and one recrystallization
in ethanol gave 100% ee diastereomer in 25 ¨C 29% overall
yield.
It?ˉs worth noting that the Pfizer group have come up
with a new process of preparing pregabalin (X) via
enantioselective reduction, that promises to further reduce
cost and waste associated with the manufacture of this drug.