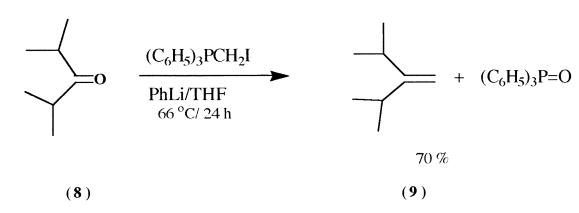
Triphenylmethylphosphonium iodide (8.55 g, 21.2 mmoles)
was weighed into a dry 100 mL round bottom flask, and 40 mL of
dry tetrahydrofuran were cannulated into the reaction vessel.
Phenyllithium in cyclohexane (11.8 mL of a 1.8 M solution, 21.2
mmoles) was added dropwise using an addition funnel. A color
change from yellow to dark brown was observed. The mixture was
stirred for 3 hrs at room temperature. 2,4-Dimethyl-3-pentanone (8) (2.4 g, 21.2 mmoles) was added slowly to the reaction
mixture resulting in a color change from brown to gray. After stirring
for an additional hour, a condenser was connected to the reaction
vessel, and the mixture was heated at reflux for 24 hours.
The mixture was allowed to cool to room temperature and
extracted successively with aqueuos of 5% HCI, 10% NaHCO3, and
distilled water. The organic layer was separated and dried over
anhydrous MgSO4, and the drying agent was removed by gravity
filtration. The solvent was removed by fractional distillation. Gas
61
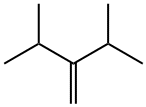
chromatographic analysis indicated the presence of 1,-
diisopropylethylene (-70%) (9), unreacted ketone (-20%) .
The distillate was purified by column chromatography on silica gel
(60-100 mesh, 2 in height and 2 in i.d.), using hexane as the eluant.
The 1,1-diisopropylethylene was identified and characterized by 1 H and 13C-NMR spectroscopy.