What is the difference between benzoic acid and formic acid?
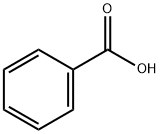
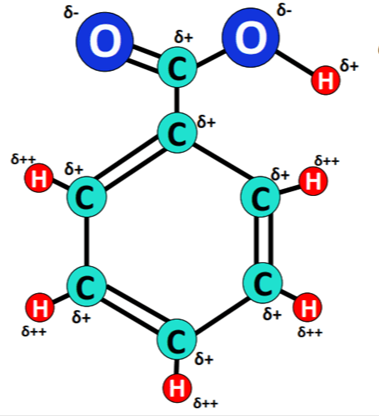
Benzoic acid is also known as benzoic acid. Its molecular formula is C6H5COOH. The simplest aromatic acid in which the carboxyl group is directly connected to the carbon atom of the benzene ring. It is a compound formed by replacing one hydrogen on the benzene ring with a carboxyl group (-COOH). It is colorless and tasteless flaky crystals. Its vapor is very irritating and can easily cause coughing after inhalation. Slightly soluble in water, easily soluble in organic solvents such as ethanol, ether, chloroform, benzene, toluene, carbon disulfide, carbon tetrachloride and turpentine.

Difference between Benzoic acid and Formic acid
Benzoic acid is a simple aromatic carboxylic acid with aromaticity and carboxylic acid properties. Therefore, two major types of chemical reactions can occur, one is the substitution reaction on the benzene ring, and the other is the carboxyl reaction.
Benzoic acid
The ionization constant of benzoic acid in water Ka=6.4×10-5 (25℃), the acidity of benzoic acid is slightly stronger than that of cyclohexanecarboxylic acid, this is because the sp2 hybrid carbon atom on the benzene ring is more electronegative The effect is weak.
Benzoic acid can be used as a preservative for food, feed, latex, and toothpaste. Under acidic conditions, it has inhibitory effects on molds, yeasts and bacteria, but has weaker effects on acid producing bacteria. The bacteriostatic activity of the molecular state of benzoic acid is higher than that of the ionic state. Therefore, when the pH is less than 4, the bacteriostatic activity is high, and the optimum pH for bacteriostasis is 2.5-4.0, generally lower than the pH value of 4.5-5.0. Due to the low solubility of benzoic acid in water, it is actually added to the appropriate amount of sodium carbonate or sodium bicarbonate, dissolved in hot water above 90 ℃, and converted into sodium benzoate before being added to food. If benzoic acid must be used, it can be dissolved with an appropriate amount of ethanol before application. Since the solubility of benzoic acid to water is lower than that of sodium benzoate, when using sodium benzoate in acidic foods, care should be taken to prevent precipitation due to the conversion of sodium benzoate to benzoic acid and reduce its use effect. Benzoic acid can be used together with p-hydroxybenzoic acid esters in soy sauce and soft drinks to increase the effect.
Formic acid
Formic acid (chemical formula HCOOH, molecular formula CH2O2, molecular weight 46.03), commonly known as formic acid, is the simplest carboxylic acid. Colorless liquid with pungent odor.
Formic acid is a weak electrolyte with a melting point of 8.6°C and a boiling point of 100.8°C. It is very acidic and corrosive, which can stimulate skin blistering. Exist in the secretions of bees, certain ants and caterpillars. It is an organic chemical raw material and also used as a disinfectant and preservative.
);You may like
Related articles And Qustion
Lastest Price from Benzoic acid manufacturers

US $1200.00/T2024-04-26
- CAS:
- 65-85-0
- Min. Order:
- 20T
- Purity:
- 99%
- Supply Ability:
- 5000kg/day

US $10.00/kg2024-04-25
- CAS:
- 65-85-0
- Min. Order:
- 1kg
- Purity:
- 99.8%
- Supply Ability:
- 10000ton