Difference Between Isopropyl Alcohol and Acetone?
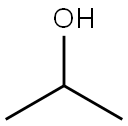
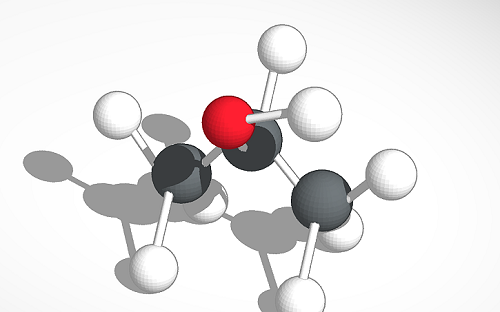
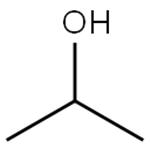
The key difference between acetone and isopropyl alcohol is that acetone has a C=O bond in the middle of the chemical structure, whereas isopropyl alcohol has a C-OH group in the middle of the chemical structure.
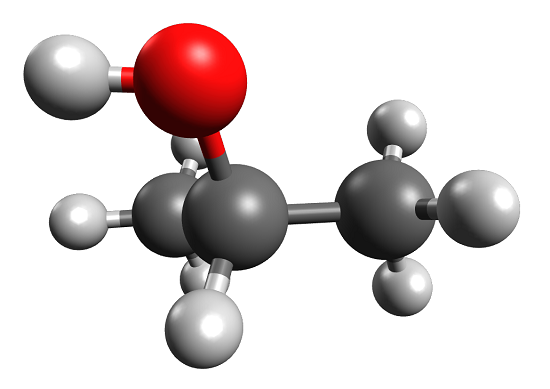
Acetone and IPA have very similar structures in their chemical formulas; both these compounds have three carbon atoms per molecule, and there are substitutions at the middle carbon. The substituted group at the middle carbon are different from each other; acetone has an oxo-group while isopropyl alcohol has a hydroxyl group.
Despite the differences in structures between acetone and IPA, both are highly soluble. They can dissolve non-polar compounds and are highly soluble in most organic solvents such as water, alcohols, ether and benzene. For this reason, IPA and acetone are widely used as solvents and cleaning agents for coating and industrial production processes.
Acetone is widely used in the labs as a solvent to clean the vials and tubes as it is good solvent for organic materials. While Isopropyl alcohol is used as a rubbing alcohol for cleaning contaminants on the body before injection. Both are good solvents for organic materials.
You may like
Related articles And Qustion
See also
Lastest Price from Isopropyl alcohol manufacturers

US $0.00-0.00/kg2025-04-21
- CAS:
- 67-63-0
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons
US $0.00/kg2025-04-15
- CAS:
- 67-63-0
- Min. Order:
- 20kg
- Purity:
- 99.0%
- Supply Ability:
- 20 tons