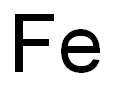What is Iron?
Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust.

Existence
In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching 1,500 °C (2,730 °F) or higher, about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia by about 2000 BCE,and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels are by far the most common industrial metals, because of their mechanical properties and low cost.
Properties
Pristine and smooth pure iron surfaces are mirror-like silvery-gray. However, iron reacts readily with oxygen and water to give brown to black hydrated iron oxides, commonly known as rust. Unlike the oxides of some other metals, that form passivating layers, rust occupies more volume than the metal and thus flakes off, exposing fresh surfaces for corrosion. Although iron readily reacts, high purity iron, called electrolytic iron, has better corrosion resistance.
Efficacy and effect
The body of an adult human contains about 4 grams (0.005% body weight) of iron, mostly in hemoglobin and myoglobin. These two proteins play essential roles in vertebrate metabolism, respectively oxygen transport by blood and oxygen storage in muscles. To maintain the necessary levels, human iron metabolism requires a minimum of iron in the diet. Iron is also the metal at the active site of many important redox enzymes dealing with cellular respiration and oxidation and reduction in plants and animals.
Chemically, the most common oxidation states of iron are iron(II) and iron(III). Iron shares many properties of other transition metals, including the other group 8 elements, ruthenium and osmium. Iron forms compounds in a wide range of oxidation states, −2 to +7. Iron also forms many coordination compounds; some of them, such as ferrocene, ferrioxalate, and Prussian blue, have substantial industrial, medical, or research applications.
You may like
Related articles And Qustion
Lastest Price from Iron manufacturers

US $100.00-75.00/kg2025-04-21
- CAS:
- 7439-89-6
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000Ton

US $1300.00-1200.00/tons2025-03-07
- CAS:
- 7439-89-6
- Min. Order:
- 10tons
- Purity:
- 99%
- Supply Ability:
- 1000tons