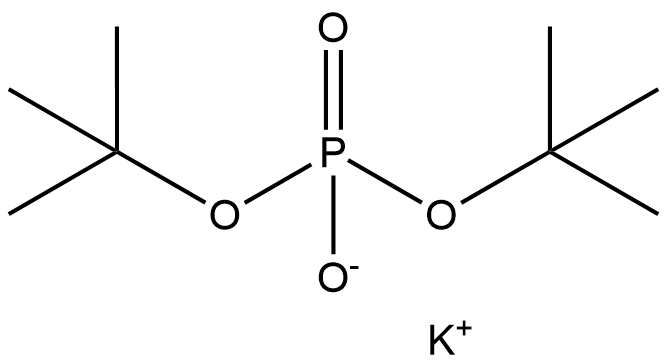
Uses of Potassium di-tert-butylphosphate as the synthetic Intermediate
Potassium di-tert-butylphosphate (Potassium di-t-butyl phosphate; Di-t-Butylphosphate Potassium; postassiumdi-tert-butylphosphate; Potassium Di-tert-butylphosphate, 95+%; Di-tert-butyl phosphate potassium salt; Phosphoric acid di-tert-butyl ester, potassium salt; C8H18KO4P) have a unique activity structure that was commonly used as raw materials and intermediates for pharmaceutical synthesis[1].
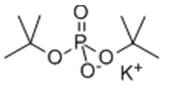
Potassium di-tert-butylphosphate can react with chloromethyl chlorosulfate in an organic solvent (dichloromethane or tetrahydrofuran) in the presence of a base (sodium carbonate or potassium carbonate) and catalyst (tetrabutylammonium sulfate or tetrabutylammonium chloride) under mild conditions for preparing chloromethyl di-tertbutylphosphate[2]. The obtained chloromethyl di-tertbutylphosphate is an nucleophile intermediate for use in preparing water soluble azole antifungal compounds, pyridone amides useful modulators of sodium channels, 2,4-pyrimidinediamine compound, substituted pyrrolidine-3-carboxamides, substituted azaindole compounds, and phosphonooxymethyl prodrugs of HIV-1 attachment inhibitors, by chloromethyl participating in substituted reaction with amino or hydroxyl of compounds[3-7].
For example, chloromethyl di-tertbutylphosphate react with 2-(4-fluor-2-methylphenoxy)-N-(2-oxo-1, 2-dihydropyridin-4-yl)-4-(trifluoromethyl) benzamide in dichloromethane and N, N–dimethylformamide for giving N-[1-(chloromethyl)-2-oxo-4-pyridyl]-2-(4-fluoro-2-methyl- phenoxy)-4-(trifluoromethyl) benzamide; Di-tert-butyl chloromethyl phosphate can react with chiral 4-((2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5- neopentylpyrrolidine-2-carboxamido)-3-methoxybenzoic acid and obtaine chiral 4-(2R,3S4R,5S)-3-(3-chloro-2-fluoro-phenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethyl-propyl)-pyrrolidie-2-carbonylamino-3-methoxy-benzoic acid di-tert-butoxy phosphoryloxymethyl ester.
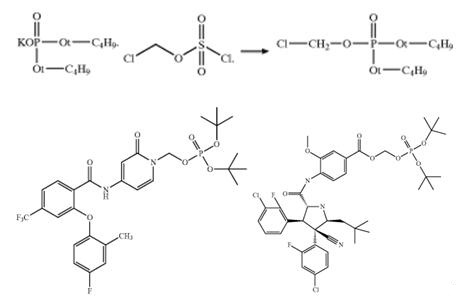
Potassium di-tert-butylphosphate can react with compounds with halohydrocarbon groups (iodo- or chloro-) by nucleophilic substitution process for preparing pyridone amides useful modulators of sodium channels, substituted pyrrolidine-2-carboxamides, and ect[7,8]. For example, potassium di-tert-butylphosphate can directly react with N-(1-(chloromethyl)-2-oxo-1, 2-dihydropyridin-4-yl) -2-(4-fluoro-2-methylphenoxy)-4-(trifluorom-continuedethyl) benzamide in ethyl acetate with TBAI and produce of di-tert-butyl([4-(2-(4-fluoro-2-methylphenoxy )-4- (trifluoromethyl)benzamido)-2-oxopyridin-1-(2H)-yl)methyl) phosphate[7]. Moreover, potassium di-tert-butylphosphate also can react with chiral 2-iodoethyl 4-((2R,3S,4R,5S)-3-(3-chloro-2- fluorophenyl)-4-4(4-chloro-2-fluorophenyl)-4-cyano-5-neopentypyrrolidine-2-carboxamido)-3-methoxybenzoate for producing 2-(di-tert-butoxyphosphoryloxy)ethyl 4-((2R,3S,4R,5S)-3- (3-chloro-2-fluorophenyl)-4-4(4-chloro-2-fluorophenyl)-4-cyano-5-neopentypyrrolidine-2-carboxamido)-3-methoxybenzoate, which is further used to prepare substituted pyrrolidine-2-carboxamides[8].
Recently, di-tert-butylphosphate complexes of metal ions, such as cobalt(II), zinc(II), Mn(II) and Cu(II) di-tert-butylphosphate complexes, which were derived from potassium di-tert-butylphosphate, could work as precursors for preparing metal phosphate materials with specific crystal structure and spectral characterization[10,11].
In conclusion, potassium di-tert-butylphosphate as pharmaceutical intermediates were widely applied to provide phosphate groups for some certain structure chemical compounds.
References
[1] https://www.chemicalbook.com/ProductChemicalPropertiesCB0709965_EN.htm.
[2] Chadwick, S., Haines, K. (2006). U.S. Patent Application No. 11/206,318.
[3] McKeever, B., Diorazio, L. J., Jones, M. F., Ferris, L., Janbon, S. L. M., Siedlecki, P. S., Crafts, P. A. (2017). U.S. Patent No. 9,695,204. Washington, DC: U.S. Patent and Trademark Office.
[4] Anderson, C., Hadida-Ruah, S. S., Golec, J. M. C., Zhang, B., Littler, B. J., Keshavarz-Shokri, A., Belmont, D. T. (2019). U.S. Patent Application No. 10/253,054.
[5] Bartkovitz, D. J., Chu, X. J., Vu, B. T., Zhao, C., Fishlock, D. (2015). U.S. Patent No. 8,993,614. Washington, DC: U.S. Patent and Trademark Office
You may like
Related articles And Qustion
See also
Lastest Price from Potassium di-tert-butyl phosphate manufacturers

US $10.00/kg2023-09-07
- CAS:
- 33494-80-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500t/month

US $0.00/KG2023-08-31
- CAS:
- 33494-80-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month