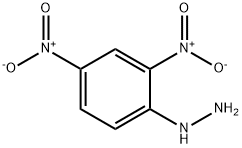
The reaction of 2,4-Dinitrophenylhydrazine to test the existing of aldehyde or ketone
General Description
2,4-Dinitrophenylhydrazine (abbreviated 2,4-DNP for short) is the chemical compound C6H3 (NO2) 2NHNH2, and it is often used to qualitatively test for carbonyl groups associated with aldehydes and ketones. The hydrazone derivatives can also be used as evidence toward the identity of the original compound. It is relatively sensitive to shock and friction; it is a shock explosive, so care must be taken with its use. To reduce its explosive hazard, it is usually supplied wet [1].
2,4-Dinitrophenylhydrazine is a compound with a benzene ring, two Nitro groups, and a hydrazine (two nitrogen atoms bonded directly to each other) functional group.
Reaction in tesing aldehyde or ketone
It is used as a Brady's reagent to test the existing of a ketone or aldehyde.
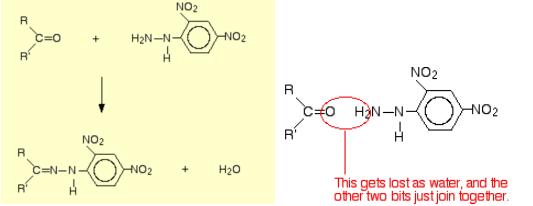
Scheme 1 The reaction of 2,4-DNP with the carbonyl group of aldehydes and ketones
As shown in scheme 1 (left), herein, R and R' can be any combination of hydrogen or hydrocarbon groups (such as alkyl groups). If at least one of them is a hydrogen, then the original compound is an aldehyde. If both are hydrocarbon groups, then it is a ketone. The product is known as a "2,4-dinitrophenylhydrazone". Notice that all that has changed is the ending from "-ine" to "-one". The product from the reaction with ethanal would be called ethanal 2,4-dinitrophenylhydrazone; from propanone, would get propanone 2,4-dinitrophenylhydrazone - and so on. That's not too difficult!
The reaction is known as a condensation reaction. A condensation reaction is one in which two molecules join together with the loss of a small molecule in the process. In this case, that small molecule is water.
In terms of mechanisms, this is a nucleophilic addition-elimination reaction. The 2,4-dinitrophenylhydrazine first adds across the carbon-oxygen double bond (the addition stage) to give an intermediate compound which then loses a molecule of water [2]. The reaction has two uses in testing for aldehydes and ketones. Firstly, can use it to test for the presence of the carbon-oxygen double bond. We only get an orange or yellow precipitate from a carbon-oxygen double bond in an aldehyde or ketone. Secondly, we can use it to help to identify the specific aldehyde or ketone.
During the test, five drops of the compound to be tested are mixed with 5 drops of the dinitrophenylhydrazine reagent (an orange solution) in 2 ml of ethanol and the tube shaken. If no positive test is observed immediately, the mixture should be allowed to stand for 15 minutes. A positive test is indicated by the formation of a yellow, orange or orange-red precipitate as shown in figure 1[3].

Figure 1 a negative test (left) and a positive test (right)
References
[1] https://en.wikipedia.org/wiki/2,4-Dinitrophenylhydrazine
[2] https://www.chemguide.co.uk/organicprops/carbonyls/addelim.html
[3] http://dept.harpercollege.edu/chemistry/chm/100/dgodambe/thedisk/qual/dnp.htm
);