Medical uses, Mechanism and Toxicities of Irinoteca
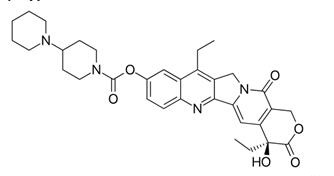
Irinotecan, sold under the brand name Camptosar among others, is an antineoplastic enzyme inhibitor primarily used in the treatment of colorectal cancer. It is a derivative of camptothecin that inhibits the action of topoisomerase I. Irinotecan prevents religation of the DNA strand by binding to topoisomerase I-DNA complex, and causes double-strand DNA breakage and cell death. It is a derivative of camptothecin. Irinotecan was approved for the treatment of advanced pancreatic cancer in October, 2015 (irinotecan liposome injection, trade name Onivyde) [1].
Common side effects include diarrhea, vomiting, bone marrow suppression, hair loss, shortness of breath, and fever. Other severe side effects include blood clots, colon inflammation, and allergic reactions. Those with two copies of the UGT1A1*28 gene variant are at higher risk for side effects. Use during pregnancy can result in harm to the baby. Irinotecan is in topoisomerase inhibitor family of medication. It works by blocking topoisomerase 1 which results in DNA damage and cell death [2].
Irinotecan was approved for medical use in the United States in 1996. Itis on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[3] In the United Kingdom itis available as a generic medication and costs the NHS about 114.00pounds per 100 mg. It is made from the natural compound camptothecin [2].
Medical uses
Irinotecan hydrochloride is an analogue of camptothecin, an extract from the Chinese tree Camptotheca acuminate, with higher aqueous solubility than camptothecin. Irinotecan was a pro-drug that is metabolically activated in the body to 7-ethyl-10hydroxycamptothecin (SN-38). Irinotecan has a broad spectrum of antitumor activity both in vitro and in vivo and is associated with more predictable and clinically manageable toxicity than the originally isolated structure. After clinical trials, irinotecan became commercially available in Japan for treatment of lung, cervical and ovarian cancers in 1994. Irinotecan was first approved for the treatment of metastatic colorectal cancer (CRC) refractory to 5-fluorouracil (5-FU) in the
United States in 1996, followed by approval in combination with 5-FU/leucovorin (LV) for the first-line treatment of metastatic CRC. A wide variety of clinical trials performed to date have revealed a survival advantage of irinotecan-based regimens in patients with metastatic CRC, making irinotecan hydrochloride one of the key drugs for the treatment of metastatic CRC. Recently, overall survival (OS) longer than 30 mo was achieved in patients with metastatic CRC who received irinotecan-based combination chemotherapy [3].
Mechanism
Irinotecan is activated by hydrolysis to SN-38, an inhibitor of topoisomerase I. This is then inactivated by glucuronidation by uridine diphosphate glucoronosyl transferase 1A1 (UGT1A1). The inhibition of topoisomerase I by the active metabolite SN-38 eventually leads to inhibition of both DNA replication and transcription. The molecular action of irinotecan occurs by trapping a subset oftopoisomerase-1-DNA cleavage complexes, those with a guanine +1 in the DNA sequence. One irinotecan molecule stacks against the base pairs flanking the topoisomerase-induced cleavage site and poisons(inactivates) the topoisomerase 1 enzyme [2].
Toxicities
In early clinical development, the dose-limiting toxicity (DLT) of irinotecan hydrochloride was found to be severe neutropenia and delayed diarrhea. Severe, occasionally life-threatening toxicity occurs sporadically, even in patients with relatively good physical condition who have a low risk of chemotherapy-induced toxicity, who are eligible for enrollment in clinical trials of anticancer drugs. Interindividual variability in the pharmacokinetics of SN-38 resulting from glucuronide formation is at least one of the major causes of irinotecan-induced severe toxicity. Investigators have thus focused primarily on the polymorphic glucuronidation of SN-38 by UDP-glucuronosyltransferase (UGT) 1A1, since UGT1A1 is the enzyme primarily involved in endogenous bilirubin glucuronidation as well as in irinotecan glucuronidation. Such studies have shown that genetic polymorphisms in the UGT1A1 gene, such as UGT1A1*28 and UGT1A1*6 are associated with irinotecan-induced severe toxicities, resulting in revision of the package inserts in the United States, Japan, and other countries, including a recommendation to use a lower initial dose of irinotecan in patients with UGT1A1*28/*28, UGT1A1*6/*6 or UGT1A1*6/*28 genotype[3].
Reference
[1] https://www.drugbank.ca/drugs/DB00762
[2] https://encyclopedia.thefreedictionary.com/Irinotecan
[3] Fujita, Ken-ichi et al. “Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer.” World journal of gastroenterology vol. 21,43 (2015): 12234-48. doi:10.3748/wjg.v21.i43.1223
You may like
See also
Lastest Price from Irinotecan manufacturers

US $0.00/kg2024-04-12
- CAS:
- 97682-44-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton
US $0.00-0.00/kg2023-11-01
- CAS:
- 97682-44-5
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 20 tons