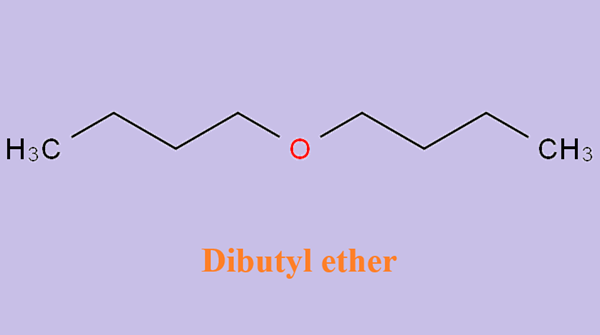
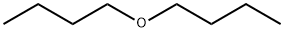Mechanism of Butyl ether
Butyl ether is used mainly as a solvent for organic materials such as resins, oils, hydrocarbons, esters, gums, and alkaloids. It is also used as an extracting agent in metal separation, as a reacting medium in organic synthesis processes, and as a solvent in teaching, research and analytical laboratories.
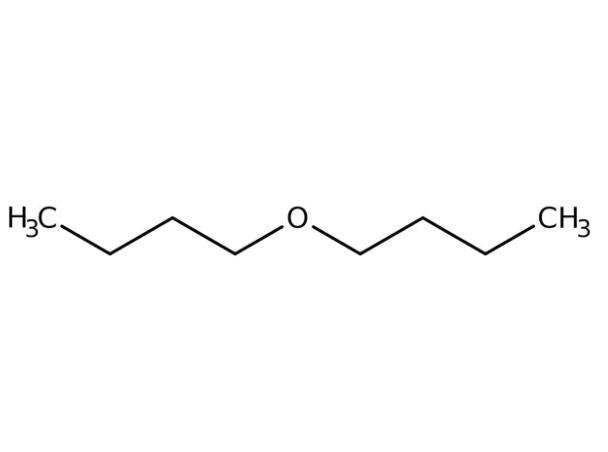
Environmental Fate
Production of butyl ether and its use as an extracting agent and a solvent may result in its release to the environment through various waste streams. If released to air, a vapor pressure of 6.0 mmHg at 25°C indicates that butyl ether will exist solely as a vapor in the ambient atmosphere. Vaporphase butyl ether reacts in the atmosphere with hydroxyl radicals; the half-life for this reaction in air has been estimated to be 13 h. Direct photolysis is not expected to be an important removal process since aliphatic ethers do not absorb light in the environmental spectrum.
If released to soil, butyl ether is expected to have high mobility based on its estimated adsorption coefficient (Koc) of 51. Volatilization from moist soil surfaces may be an important fate process based on its reported Henry’s law constant of 6.0×10-3 atm m3 mol-1. Butyl ether is expected to volatilize from dry soil surfaces based on its reported vapor pressure. If released into water, butyl ether is not expected to adsorb to suspended solids and sediment in water based on its Koc. Aqueous screening studies indicate biodegradation may be an important fate process in both soil and water; for example, butyl ether reached 3–4% of its theoretical biological oxygen demand (BOD) over 4 weeks using an activated sludge seed. Volatilization from water surfaces is expected to occur based on this compound’s estimated Henry’s law constant. Estimated volatilization half-lives for a model river and model lake have been reported to be 3.5 h and 4.6 days, respectively. Bioconcentration factors (BCFs) ranging from 30 to 114 in carp suggest that bioconcentration in aquatic organisms is moderate to high. Butyl ether is not expected to undergo hydrolysis in the environment due to the lack of hydrolyzable functional groups.
Mechanism
Butyl ether has the ability to dissolve lipids. As a result, it causes irritation and pain on contact with the eyes and nasal mucosa. It also causes dermal irritation and dermatitis on contact with the skin. Damage caused by butyl ether appears to be scattered loss of epithelial cells due to solution of phospholipid cell membranes. At the central nervous system (CNS) level, butyl ether, like other volatile organic solvents, depresses the CNS by dissolving in the lipid membrane of the cells and disrupting the lipid matrix. These effects are known as membrane fluidization. At the molecular level, membrane fluidization disrupts solute gradient homeostasis, which is essential for cell function.
You may like
Related articles And Qustion
Lastest Price from Dibutyl ether manufacturers

US $0.00/KG2025-04-21
- CAS:
- 142-96-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $30.00-10.00/KG2025-04-15
- CAS:
- 142-96-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg