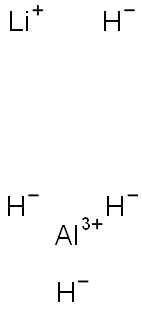Lithium aluminium hydride-Hazard and Toxicity
Description
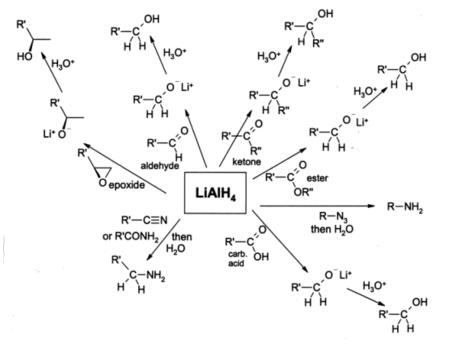
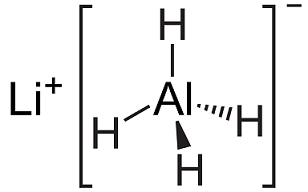
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, especially for the reduction of esters, carboxylic acids, and amides. The solid is dangerously reactive toward water, releasing gaseous hydrogen (H2). Some related derivatives have been discussed for hydrogen storage.
Lithium aluminium hydride is a powerful reducing agent. React violently on contact with many oxidizing agents. Ignites by friction, especially if powdered. Reacts vigorously with hydroxy compounds such as water, alcohols, carboxylic acids.
Toxicity Data
TLV-TWA (ACGIH) 2 mg (Al)/m3
Major Hazards
Reacts violently with water, acids, and many oxygenated compounds; may ignite in moist air; corrosive to skin, eyes, and mucous membranes. Lithium aluminum hydride is a flammable substance. It ignites spontaneously on grinding and reacts violently with water and many organic substances. Diethyl ether, tetrahydrofuran or another suitable solvent should be used in its synthetic applications. Dry or powdered limestone is an appropriate fire extinguishing agent.
Toxicity
Lithium aluminum hydride is highly corrosive to the skin, eyes, and mucous membranes. Contact with moisture forms lithium hydroxide, which can cause severe burns. Powdered LAH forms dusts that can pose an inhalation hazard. Ingestion of this substance may cause aching muscles, nausea, vomiting, dizziness, and unconsciousness and may be fatal. Ingestion can result in gas embolism due to the formation of hydrogen. No chronic effects of lithium aluminum hydride have been identified.
Flammability and Explosibility
Lithium aluminum hydride is a highly flammable solid and may ignite in moist or heated air. Exposure to water results in the release of hydrogen, which can be ignited by the heat from the exothermic reaction. Lithium aluminum hydride should not be used as a drying agent for solvents because fires can easily result (LAH decomposes at about 125° C, a temperature easily reached at a flask's surface in a heating mantle). The decomposition products of LAH can be quite explosive, and the products of its reaction with carbon dioxide have been reported to be explosive. Use dry chemical powder or sand to extinguish fires involving lithium aluminum hydride. Never use water or carbon dioxide extinguishers on an LAH fire.
Reactivity and Incompatibility
Lithium aluminum hydride reacts violently with water, acids, oxidizers, alcohols, and many oxygenated organic compounds, including, in particular, peroxides, hydroperoxides, and peracids. LAH reacts with many metal halides to produce metal hydride products, which are flammable and toxic.
Storage and Handling
In particular, LAH should be handled in areas free of ignition sources under an inert atmosphere. Safety glasses, impermeable gloves, and a fire-retardant laboratory coat are required. A dry powder fire extinguisher or pail of sand (and shovel) must be available in areas where LAH is to be handled or stored. Work with large quantities of powdered LAH should be conducted in a fume hood under an inert gas such as nitrogen or argon.
Lithium aluminum hydride should be stored in tightly sealed containers in a cool, dry area separate from combustible materials. Dry LAH powder should never be exposed to water or moist air. Lithium aluminum hydride can be a finely powdered reagent that produces a reactive dust on handling. The older practice of grinding lithium aluminum hydride prior to use can cause explosions and should not be employed.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If lithium aluminum hydride is ingested, obtain medical attention immediately. If large amounts of LAH dust are inhaled, move the person to fresh air and seek medical attention at once.
In the event of a spill, instruct others to maintain a safe distance; while wearing a face shield and goggles, laboratory coat, and butyl rubber gloves, cover the spilled material with sand. Scoop the resulting mixture into a container suitable for treatment or disposal as discussed below.
Disposal
Small amounts of excess LAH can be destroyed by forming a suspension or solution in an inert solvent such as diethyl ether or hexane, cooling in an ice bath, and slowly and carefully adding ethyl acetate dropwise with stirring. This is followed by the addition of a saturated aqueous solution of ammonium chloride.
Excess lithium aluminum hydride and the products of the treatment described above should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
You may like
Related articles And Qustion
Lastest Price from Lithium Aluminum Hydride manufacturers

US $10.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $0.00-0.00/kg2025-04-21
- CAS:
- 16853-85-3
- Min. Order:
- 1kg
- Purity:
- 99.99%
- Supply Ability:
- 20 tons