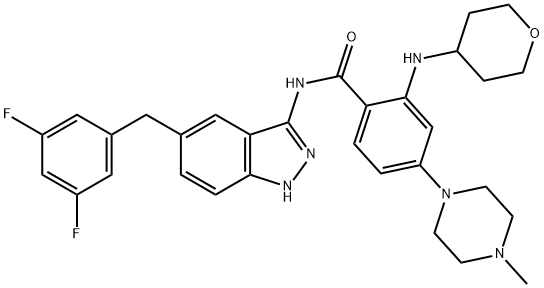
Entratinib's mechanism of action
Background and overview[1][2]
Entritinib was approved for marketing in Japan on June 19, 2019, and was approved by the FDA on August 15, 2019 for the treatment of advanced recurrent solid tumors with positive neurotrophic tyrosine receptor kinase (NTRK) fusion Adult and pediatric patients; and for the treatment of metastatic non-small cell lung cancer with mutations in the ROS1 gene. Entritinib is a new type of oral tyrosine kinase inhibitor (TKI) with central nervous system activity, targeted to treat solid tumors carrying NTRK1/2/3, ROS1 and ALK gene fusion mutations. The only TRK inhibitor clinically proven to be effective against primary and metastatic CNS diseases and has no adverse off-target activity. Entratinib is the first drug approved in Japan to target NTRK gene fusion tumors, and is approved for use in a series of difficult-to-treat solid tumor types, including pancreas, thyroid, salivary glands, breast, colorectal and lung cancer.
Clinical data[1]
Including the key trials of Phase II STARTRK-2, Phase I STARTRK-1 and Phase I ALKA-372-001 trials, as well as data from the Phase I/II STARTRK-NG study of pediatric patients.
The results show that:
In the pivotal Phase II STARTRK-2 study, Entratinib reduced tumors in patients with NTRK fusion-positive solid tumors by more than half (objective response rate [ORR]=56.9%). Objective responses to Entratinib (secondary endpoint, median duration of response [DoR] = 10.4 months) were observed in 10 different solid tumor types, including people with and without brain metastases at baseline.
Importantly, Entratinib also shrinks tumors in patients with brain metastases, and tumors that have spread to the brain in more than half of the population (intracranial response [IC]ORR=50.0%).
In the STARTRK-NG study, Entratinib also reduced tumors in children and adolescents with NTRK fusion-positive solid tumors, including patients with primary central nervous system tumors.
Roche also updated the latest clinical data of entritinib at this year’s ASCO conference, showing that 54 patients with NTRK gene fusion had an ORR of 57%, of which 42 patients without brain metastases had an ORR of 59.5%, and 12 had brain The ORR of patients with metastases was 50%; the ORR of 53 patients with ROS1 gene fusion was 77%, of which 30 patients without brain metastases had an ORR of 80%, and 23 patients with brain metastases had an ORR of 73.9%.
Mechanism of action [1]
ALK, ROS1 and NTRK1/2/3 gene fusion is the pathogenic factor of a variety of hematological and solid malignancies. ALK gene fusion occurs in 3% to 4% of non-small cell lung cancer (non-small cell lung cancer, NSCLC), in anaplastic large cell lymphoma (ALCL), renal cell tumor, breast cancer, colorectal cancer and esophagus Cancer and other types of cancer also occur. There are currently four targeted ALK therapies (crizotinib, ceritinib, alectinib and brigatinib) approved for use in ALK recombinant-positive lung cancer patients. The incidence of ROS1 gene fusion in non-selective NSCLC is 1% to 2%, and it also occurs in tumors such as glioblastoma and cholangiocarcinoma. Crizotinib is approved for ROS1 recombinant lung cancer. Compared with traditional chemotherapy, objective response rate (ORR) and progression free survival (PFS) have improved.
Tropomyosin-related kinase (Trk) protein is a type of nerve growth factor receptor, which plays an important role in the development and function of neurons in the central and peripheral nervous system. Its family consists of three subtypes of high homology, TrkA (tropomyosin-relatedkinaseA), TrkB, and TrkC, which are respectively encoded by neurotrophic receptor tyrosine kinase 1 (neurotrophicreceptorkinase1, NTRK1), NTRK2 and NTRK3 genes. NTRK gene fusion occurs in a variety of adult and child solid tumors.
In common cancers (such as NSCLC, colorectal cancer), the incidence of NTRK gene fusion is relatively low, roughly 1% to 3%. However, in some rare cancers, such as infant fibrosarcoma, breast-like secretory carcinoma, and breast secretory carcinoma, the incidence of NTRK gene fusion can reach more than 90%. When Trk is activated by phosphorylation, it can affect cell proliferation, differentiation, metabolism and apoptosis through downstream pathways. Because NTRK genes are prone to rearrangement and cause gene fusion, this chromosomal mutation will lead to abnormal regulation of the downstream signaling pathway of Trk kinase, and excessive activation of the pathway can lead to cancer. Entratinib is a kinase inhibitor that targets the fusion of ALK, ROS1 and NTRK genes. It inhibits the kinase catalytic activity by competing with ATP for binding sites, thereby achieving the therapeutic effect of inhibiting tumors. It has a late stage of the fusion of ALK, ROS1 and NTRK genes. Or metastatic tumors have strong anti-cancer potential.
Pre-clinical pharmacodynamics[1]
Preclinical studies have shown that Entratinib has shown strong anti-tumor activity in genetically engineered mutant mouse tumor cell lines and human xenograft (PDX) tumor models. In in vitro tumor cell lines, compared with the blank control group, low concentrations of Entratinib can strongly inhibit the activities of TrkA/B/C, ROS1 and ALK. The average median inhibitory concentrations (IC50) for TrkA/B/C enzymes were 0.002, 0.0005 and 0.0011 μmol·L-1, respectively; the IC50 for ROS1 was 0.007 μmol·L-1; for ALK IC50 is 0.019 μmol·L-1. In the KM-12 colorectal cancer cell line with TPM3-NTRK1 gene fusion, Entritinib can inhibit cell proliferation, affect cell cycle arrest and apoptosis, and can also cause downstream genes (such as AKT, ERK and PLCγ1) to inactivate .
Entritinib can inhibit the proliferation of colorectal cancer, lung cancer, ALCL and neuroblastoma cell lines fused with ALK gene. In vivo PDX model mouse tumor research, including colorectal cancer model with TPM3-NTRK1 gene fusion, soft tissue sarcoma model with TPM3-NTRK1 gene fusion, MPRIP-NTRK1, ETV6-ROS1, SCD4-ROS1 and CD74-ROS1 ALK fusion lung cancer Model, ETV6-NTRK3 gene fusion head and neck cancer model and ETV6-NTRK3 gene fusion acute myeloid leukemia. By measuring the tumor volume of the control group and the test group, calculate the tumor inhibition percentage (%TI), the calculation formula is TI(%)=100-(average tumor volume of the treatment group/average tumor volume of the control group)×100, and the results show that Tratinib has anti-tumor activity and induces tumor regression.
Complete tumor regression was observed in the Karpas-299 and SR-786PDX models of NPM1-ALK gene fusion. In the SY5Y-TRKB fusion PDX gangliocytoma model caused by the overexpression of TRKB, Entritinib inhibited tumor growth and the tumor subsided substantially. Brain metastasis of tumor cells is one of the reasons for the clinical recurrence of NSCLC. For the mouse model of intracranial injection of NSCLC tumor cell line NCI-H2228, the tumor suppressing effect of entritinib is positively correlated with the dose, while the control drug crizotinib is due to low Blood-brain permeability has no anti-tumor activity in this model.
Pharmacokinetics [1]
The pharmacokinetics of Entratinib has been studied in pre-clinical trials. At present, all clinical treatment regimens use Entritinib within 30 minutes after a meal. The results show that Entritinib is a drug with a high plasma protein binding rate. The plasma protein binding rate in humans is about 99.5%, and it is easy to wear. Cross the blood-brain barrier. In CD1 mice in vivo experiments, the average absolute bioavailability of entritinib was 77%, and the brain exposure (Cmax and AUC) was about 20% of the plasma exposure after a single dose. The peak time (Tmax) of the drug concentration in the fasted state is 2 to 4 hours, and the Tmax is about 5 to 7 hours in the fed state.
Entritinib is mainly metabolized by cytochrome P3A4 (CYP3A4), and it can be metabolized by CYP2C9 and CYP1C19 in a small amount. When clinical monitoring and the combined application of multiple drugs, it is necessary to pay attention to potential drug interactions and adjust the dosage in time. For example, the combined use of strong CYP3A inhibitors such as itraconazole may increase the plasma concentration of entretinib, which may lead to an increase in the incidence of adverse reactions. Therefore, the simultaneous use of strong CYP3A inhibitors should be avoided or reduced. When combined with a strong CYP3A4 inducer, it may reduce the plasma concentration of entretinib, thereby reducing the efficacy of entretinib, so the combined use or increasing the dose of entretinib should be avoided. When entritinib is used in combination with CYP3A4 sensitive substrates, its plasma concentration may increase, thereby increasing the incidence or severity of adverse reactions. If it cannot be avoided, the patient's adverse drug reactions should be monitored.
Security [2]
Entinib is well tolerated. Dose limiting toxicities are increased creatinine, dysgeusia, fatigue and pulmonary edema. In addition, unlike other drugs, weight gain is another noteworthy adverse event. These early findings indicate that Entinib has great promise for those with specific gene fusions. The most common adverse reactions include constipation, taste changes (dysgeusia), diarrhea, dizziness, fatigue, swelling (edema), weight gain, anemia, increased blood creatinine, shortness of breath (dyspnea) and nausea.
References
[1] Wang Jiyuan, Li Liang, Qin Yan. Research on entrectinib, a selective kinase inhibitor targeting NTRK, ROS1 and ALK fusion-positive solid tumors [J]. Chinese Journal of New Drugs, 2019, 28(19): 2360-2366.
[2] Rozlytrek (entrectinib), a broad-spectrum anticancer drug, was officially launched in Japan, Wanbang Xingjian International Medicine
You may like
Related articles And Qustion
Lastest Price from Entrectinib manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 1108743-60-7
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month

US $0.00-0.00/g2024-11-01
- CAS:
- 1108743-60-7
- Min. Order:
- 1g
- Purity:
- 98%
- Supply Ability:
- 100kgs