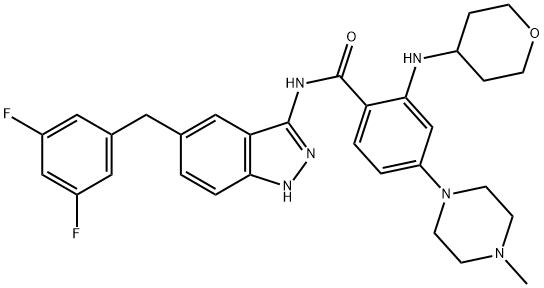
How to synthesize Entrectinib
Description
Entrectinib is an orally available, CNS-active, selective inhibitor of the tyrosine kinases tropomyosin receptor kinase (Trk) A/B/C, anaplastic lymphoma kinase (ALK), and c-ros oncogene 1 (ROS1). These gene fusions are all known to be involved in a wide variety of solid tumors, with some further complicated by brain metastases.
Entrectinib was first approved in Japan for treating adult and pediatric patients with neurotrophin receptor tyrosine kinase (NTRK) fusion-positive, advanced, or recurring solid tumors. The drug is one of a variety of next-generation tyrosine kinase inhibitors (TKIs) and received accelerated approval by the USFDA for adults with this same indication and ROS1-positive metastatic non-small cell lung cancer (NSCLC). Approval of the drug was based on several clinical trials of entrectinib in adults, one of which showed an overall response rate (ORR) of 57% in dosed patients possessing a variety of NTRK fusion-positive solid tumors. Additionally, clinical trials of adults with ROS1-rearranged NSCLC showed a 78% ORR after treatment with entrectinib.
Synthesis method
A route for the preparation of entrectinib has been reported by Nerviano that relies upon joining functionalized carboxylic acid fragment 258 with 5-(3,5-difluorobenzyl)-1H-indazol-3- amine (259).
Synthesis of Entrectinib Benzoic Acid Intermediate 258
Acid fragment 258 was prepared on a gram scale by a five-step process starting from tert-butyl 4-fluoro-2-nitrobenzoate (253) (shown below). A reaction with N-methylpiperazine (254) at room temperature gave the expected substitution product a high yield. Reduction of the nitro group followed by reductive amination with pyranone 256 provided 257 in good yield. Trifluoroacetylation followed by removal of the tert-butyl ester delivered acid 258 as the trifluoroacetate salt, which was isolated by trituration from diethyl ether.
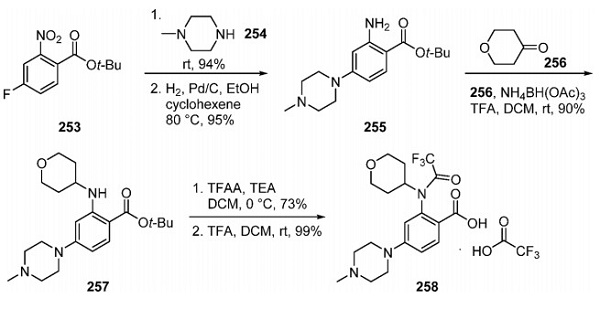
Synthesis of Entrectinib
Synthesis of the final drug product on a kilogram scale started with 258, which was converted to the corresponding acyl chloride by treatment with oxalyl chloride in DCM. The subjection of the crude acyl chloride to 259 and pyridine at −30 to −40 °C provided the core structure of entrectinib, which was obtained with >95% purity after trituration from a mixture of solvents. Cleavage of the trifluoroacetate with K2CO3 in aqueous MeOH at 10 °C, filtration, and precipitation provided amorphous entrectinib. Recrystallization of amorphous entrectinib from EtOH/water provided the desired crystal form of the drug in 66% overall yield for the three-step process.
References
[1] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
Related articles And Qustion
See also
Lastest Price from Entrectinib manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 1108743-60-7
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month

US $0.00-0.00/g2024-11-01
- CAS:
- 1108743-60-7
- Min. Order:
- 1g
- Purity:
- 98%
- Supply Ability:
- 100kgs