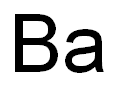Barium Industrial Applications
Barium is a silvery-white metal. It exists in nature only in ores containing mixtures of elements. The important combinations are the peroxide, chloride, sulphate, carbonate, nitrate, and chlorate. The pure metal oxidises readily and reacts with water emitting hydrogen. It combines with other chemicals such as sulphur or carbon and oxygen to form barium compounds. Barium compounds are used by the oil and gas industries to make drilling muds. Barium attacks most metals with the formation of alloys; iron is the most resistant to alloy formation. Barium forms alloys and intermetallic compounds with lead, potassium, platinum, magnesium, silicon, zinc, aluminium, and mercury. Barium compounds exhibit close relationships with the compounds of calcium and strontium, which are also alkaline earth metals. Twenty-five barium isotopes have been identified, 138Ba being the most abundant, and the others are unstable isotopes with half-lives ranging from 12.8 days for 140Ba to 12 s for 143Ba. Two of these isotopes, 131Ba and 139Ba, are used in research as radioactive tracers. The general population is exposed to barium through air, drinking water, and food.

The word barium comes from the Greek word baryos, meaning heavy, owing to the high density of its main mineral, barite. Barium, chemically resembling calcium, is, compared to other alkaline-earth metals, a relatively dense, soft, malleable, silvery- white metal like lead when freshly cut, but it readily tarnishes in moist air, becoming yellowish due to the formation of an oxide. It has a body-centered cubic crystalline structure and decomposes water, evolving hydrogen and forming the hydroxide Ba(OH)2. Barium fines, such as powder, dust, and turnings, are pyrophoric, i.e., they ignite spontaneously in air. The metal should be kept in mineral oil such as petroleum or other suitable oxygen-free liquid to exclude air. It is also decomposed by ethanol. Naturally occurring barium is a mixture of seven stable isotopes.
Barium was first identified in the mineral barite by the Swedish chemist Scheele in 1774. The pure element was first prepared in 1808 by British chemist Sir Humphry Davy, who produced barium amalgam by electrolyzing an aqueous solution of barium chloride using a liquid-mercury cathode. After distilling mercury from the barium amalgam formed, he obtained pure barium metal.
General Properties
The barium content in the lithosphere is ca. 500 mg/kg (i.e., ppm wt.), but, owing to its chemical reactivity, the metal does not occur free in nature. The chief barium-containing minerals are the sulfate barite or heavy spar [BaSO4, orthorhombic] and the carbonate witherite [BaCO3, orthorhombic].
Barium is a silvery-white metal. It exists in nature only in ores containing mixtures of ele- ments. The important combinations are peroxide, chloride, sulfate, carbonate, nitrate, and chlorate. The pure metal oxidizes readily and reacts with water, emitting hydrogen. It com- bines with other chemicals such as sulfur or carbon and oxygen to form barium compounds. Barium compounds are used by the oil and gas industries to make drilling muds. Barium attacks most metals with the formation of alloys; iron is the most resistant to alloy formation. Barium forms alloys and intermetallic compounds with lead, potassium, platinum, mag- nesium, silicon, zinc, aluminum, and mercury. Barium compounds exhibit close relation- ships with the compounds of calcium and strontium, which are also alkaline earth metals. Doctors sometimes use barium sulfate to perform medical tests and to take x-rays of the gastrointestinal tract.
Industrial Applications
After mining, barite is finely crushed and ground and undergoes a flotation beneficiation process to concentrate and separate barite from byproduct minerals (e.g., quartz). Then the concentrate ore is reduced by pyrolysis in a kiln to barium sulfide (i.e., BaS or black ash). The black ash is then dissolved in pure water, and the aqueous solution is treated with sodium carbonate to precipitate the barium-carbonate crystals. After the witherite crystals are removed and dried, the carbonate undergoes calcination, evolving carbon dioxide and giving anhydrous barium oxide, BaO. Barium metal could be obtained either by thermal reduction of the previously obtained barium oxide with molten aluminum in a vacuum or by fused barium chloride electrolysis.
The metal is used as a getter in vacuum tubes. Lithopone, a pigment containing barium sulfate and zinc sulfide, has good covering power and does not darken in the presence of sulfides. The sulfate, as permanent white, is also used in paint, in X-ray diagnostic work, and in glassmaking. Barite is extensively used as a weighing agent in oil-well drilling fluids and is used in making rubber. The carbonate has been used as a rat poison, while the nitrate and chlorate give colors in pyrotechnics. The impure sulfide phosphoresces after exposure to light. All barium compounds that are water or acid soluble are poisonous.