Applications of Prilocaine
Prilocaine is a kind of needle-like crystals with the melting point being 37-38 ℃ and the boiling point being 159-162 ℃ (0.133kPa), and refractive index (nD20) being 1.5299. Its hydrochloride ([1786-81-8]) is a white crystalline powder. The Melting point is 167-168 ℃. Prilocaine is soluble in water and ethanol, slightly soluble in chloroform. Prilocaine has sour taste and bitter taste and is odorless.
Prilocaine is an amino acid amide in which N-propyl-DL-alanine and 2-methylaniline have combined to form the amide bond; used as a local anaesthetic. Prilocaine has a role as a local anaesthetic and an anticonvulsant. Prilocaine is an amino acid amide and a monocarboxylic acid amide.
Prilocaine is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils Löfgren. In its injectable form, it is often used in dentistry. Prilocaine is also often combined with lidocaine as a topical preparation for dermal anesthesia, for treatment of conditions like paresthesia. As Prilocaine has low cardiac toxicity, it is commonly used for intravenous regional anaesthesia.
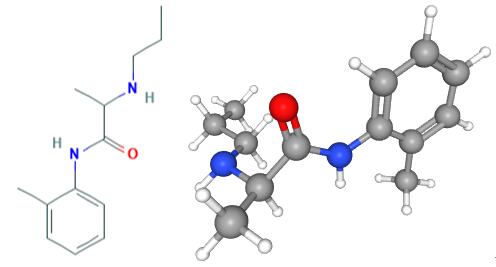
Fig 1. Chemical structure formula and three-dimensional structure of Prilocaine
Prilocaine binds to the intracellular surface of sodium channels which blocks the subsequent influx of sodium into the cell. Action potential propagation and never function is, therefore, prevented. This block is reversible and when the drug diffuses away from the cell, sodium channel function is restored and nerve propagation returns. Prilocaine is a toluidine derivative and intermediate-acting amino amide with local anesthetic property. Prilocaine stabilizes the neuronal membrane by preferential binding to and inhibiting depolarization of the voltage-gated sodium channel. This results in a decrease in membrane permeability and subsequent inhibition of the ionic sodium influx required for the initiation and conduction of impulses[1-3].
Prilocaine is synthesized by reacting 2-aminotoluene with 2-bromopropionyl chloride and then with n-propylamine. /Hydrochloride/[4].
Prilocaine has a clinical profile similar to lidocaine and is used for infiltration, peripheral nerve blocks, and spinal and epidural anesthesia[5]. Prilocaine is an amide-type LA of fast onset and intermediate duration of action. Prilocaine metabolism is similar to that of lidocaine, being rapidly metabolized by the liver and excreted in the urine as o-toluidine[6].
References
[1] Overington JP, Al-Lazikani B, Hopkins AL: How many drug targets are there? Nat Rev Drug Discov. 2006 Dec;5(12):993-6.
[2] Imming P, Sinning C, Meyer A: Drugs, their targets and the nature and number of drug targets. Nat Rev Drug Discov. 2006 Oct;5(10):821-34.
[3] Chen X, Ji ZL, Chen YZ: TTD: Therapeutic Target Database. Nucleic Acids Res. 2002 Jan 1;30(1):412-5.
[4] Gerhartz, W. (exec ed.). Ullmann's Encyclopedia of Industrial Chemistry. 5th ed.Vol A1: Deerfield Beach, FL: VCH Publishers, 1985 to Present., p. VA15-419.
[5] Suzuko Suzuki, . Kai Kuck, in Pharmacology and Physiology for Anesthesia (Second Edition), 2019.
[6] Stefanie Hultzsch, Asher Ornoy, in Drugs During Pregnancy and Lactation (Third Edition), 2015.
Related articles And Qustion
Lastest Price from Prilocaine manufacturers

US $5.00-0.50/KG2025-06-05
- CAS:
- 721-50-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS

US $5.00-0.50/KG2025-05-07
- CAS:
- 721-50-6
- Min. Order:
- 1KG
- Purity:
- 99% hplc
- Supply Ability:
- 500TONS