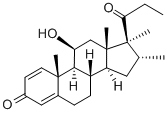
Rimexolone, an ophthalmic corticosteroid, was launched in 1995 in the U.S.A. for
the treatment of postoperative inflammation following ocular surgery and anterior
uveitis. Rimexolone has high corticoid receptor affinity and is a potent local
antiinflammatory agent with minimal systemic effects and virtually no atrophogenic
action in many animal models studied, unique among a wide range of topical steroids.
Rapid onset, long duration of action plus a superior safety profile are characteristics of
rimexolone. It was also approved in Europe for the treatment of rheumatoid arthritis and
is currently in clinical trials for tendinitis and osteoarthritis.