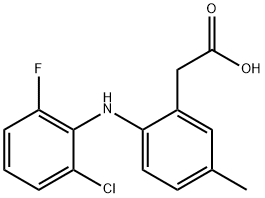
As a second-generation, selective cyclooxygenase (COX-2) inhibitor, lumiracoxib
is devoid of the gastrointestinal issues that plague other non-selective, nonsteroidal,
anti-inflammatory drugs (NSAIDs) that crossover to COX-1. As an inhibitor
of the inducible COX-2 that is up-regulated in pathological processes of
pain and inflammation, lumiracoxib blocks the conversion of arachidonic acid to
prostaglandins, the mediators of the pathological effects. It’s mode of binding to
COX-2 has been found to differ from the other selective COX-2 inhibitors; the
carboxylic acid forms hydrogen bonds with Tyr-385 and Ser-530 in the catalytic site
rather than seeking interactions within the larger hydrophobic side pocket. Since lumiracoxib is mainly metabolized by CYP2C9, a study evaluating
the co-administration of lumiracoxib with fluconazole, a potent inhibitor of
CYP2C9, was conducted, and it concluded that there was no need for lumiracoxib
dose adjustment, since changes in the systemic exposure were not significant. No
serious adverse effects were reported, but in the small number of cases where
treatment was discontinued, Gastro intestinal (GI) and musculoskeletal complaints
were common.