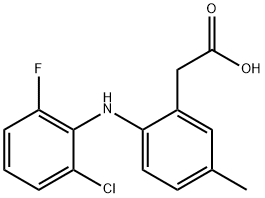
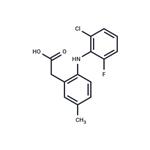
Description
As a second-generation, selective cyclooxygenase (COX-2) inhibitor, lumiracoxib
is devoid of the gastrointestinal issues that plague other non-selective, nonsteroidal,
anti-inflammatory drugs (NSAIDs) that crossover to COX-1. As an inhibitor
of the inducible COX-2 that is up-regulated in pathological processes of
pain and inflammation, lumiracoxib blocks the conversion of arachidonic acid to
prostaglandins, the mediators of the pathological effects. It’s mode of binding to
COX-2 has been found to differ from the other selective COX-2 inhibitors; the
carboxylic acid forms hydrogen bonds with Tyr-385 and Ser-530 in the catalytic site
rather than seeking interactions within the larger hydrophobic side pocket. Since lumiracoxib is mainly metabolized by CYP2C9, a study evaluating
the co-administration of lumiracoxib with fluconazole, a potent inhibitor of
CYP2C9, was conducted, and it concluded that there was no need for lumiracoxib
dose adjustment, since changes in the systemic exposure were not significant. No
serious adverse effects were reported, but in the small number of cases where
treatment was discontinued, Gastro intestinal (GI) and musculoskeletal complaints
were common.
Description
Lumiracoxib is a selective inhibitor of COX-2 with IC
50 values of 0.13 and 67 μM for COX-2 and COX-1, respectively, in isolated human whole blood. It reduces increases in the levels of prostaglandin E
2 (PGE
2; ) induced by IL-1β in human dermal fibroblasts (IC
50 = 0.14 μM), as well as in LPS-stimulated RAW 264.7 cells when used at concentrations ranging from 1 to 100 μM., Lumiracoxib (3 and 10 mg/kg) also decreases LPS-induced increases in the levels of PGE
2 in a rat model of air pouch inflammation. It reduces
M. tuberculosis-induced increases in hind paw volume and the radiological and histopathological severity of arthritic lesions in a rat model of chronic arthritis when administered at a dose of 2 mg/kg.
Chemical Properties
Pale Yellow Solid
Originator
Novartis AG (Switzerland)
Uses
Lumiracoxib is a selective cyclooxygenase-2-(COX-2) inhibitor and an anti-inflammatory agent (1,2,3,4).
Uses
antiinflammatory, analgesic, antiarthritic
Uses
Selective cyclooxygenase-2-(COX-2) inhibitor. Anti-inflammatory.
Definition
ChEBI: An amino acid that is phenylacetic acid which is substituted at position 2 by the nitrogen of 2-chloro-6-fluoroaniline and at position 5 by a methyl group. A highly selective cyclooxygenase 2 inhibitor, it was briefly used for the treatment of osteoarthrit
s, but was withdrawn due to concersns of hepatotoxicity.
Biochem/physiol Actions
Lumiracoxib (COX189) is an orally active, potent and selective cyclooxygenase-2 inhibitor (Ki = 60 nM/COX-2 vs. 3.2 μM/COX-1) that inhibits COX-2-mediated PGE2 production in human whole blood (IC50 = 130 nM; stimulation = 50 μM A23187), but not COX-1-dependent TxB2 production (IC50 = 67 μM; stimulation = 10 μg/mL LPS). Lumiracoxib shows in vivo anti-inflammatory efficacy against carrageenan-induced paw oedema (ED30 = 0.35 mg/kg p.o.), CFA-induced hyperalgesia (ED30 = 5.1 mg/kg p.o.), as well as adjuvant-induced arthritis (ED50 = 3 mg/kg/day p.o.) in rats in vivo.