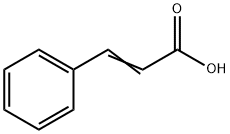
Cinnamic acid is a white crystalline organic acid, which is slightly soluble in water.
It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter and is the best indication of its environmental history and post-extraction conditions. It can also be made synthetically.
Cinnamic acid is used in flavors, synthetic indigo, and certain pharmaceuticals, though its primary use is in the manufacturing of the methyl, ethyl, and benzyl esters for the perfume industry. Cinnamic acid has a honey- like odor; it and its more volatile ethyl ester (ethyl cinnamate) are flavor components in the essential oil of cinnamon, in which related cinnamaldehyde is the major constituent. Cinnamic acid is also part of the biosynthetic shikimate and phenyl propanoid pathways. Its biosynthesis is performed by action of the enzyme phenylalanine ammonia - lyase (PAL) on phenylalanine.
Cinnamic acid is freely soluble in benzene, diethyl ether, acetone, and it is insoluble in hexane.
Cinnamic acid is also a kind of self-inhibitor produced by fungal spore to prevent germination.