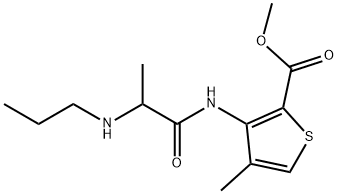
Articaine [4-methyl-3-(2-propylaminopropionamido) thiophene-2-carboxylic acid methyl ester
hydrochloride] has been widely used in dentistry since its approval by the U.S. FDA in the year
2000 because of its quick onset and short duration of action. The structure of articaine differs
from those of all other amino amide-type local anesthetics in that it contains a thiophene ring
instead of a benzene ring and a carbomethoxy group. This renders the molecule more lipophilic
and, thus, easier to cross any lipoidal membranes.
Its local anesthetic potency is approximately 1.5-fold that of lidocaine, even though it has similar
pKa (7.8) and plasma protein binding (76%) properties. Articaine also is metabolized primarily by
plasma cholinesterases because of the presence of an ester group and, therefore, has a much
shorter duration of action than lidocaine (i.e., only approximately one-fourth that of lidocaine).
Articaine undergoes rapid hydrolysis of the carbomethoxy group to give articainic acid, which is
eliminated either unchanged (75%) or as its glucuronides (25%). Compared with to other short�acting, amino amide-type local anesthetics, such as mepivacaine, lidocaine, or prilocaine,
articaine is said to be a much safer drug for regional anesthesia and is the drug of choice for
dental procedures.