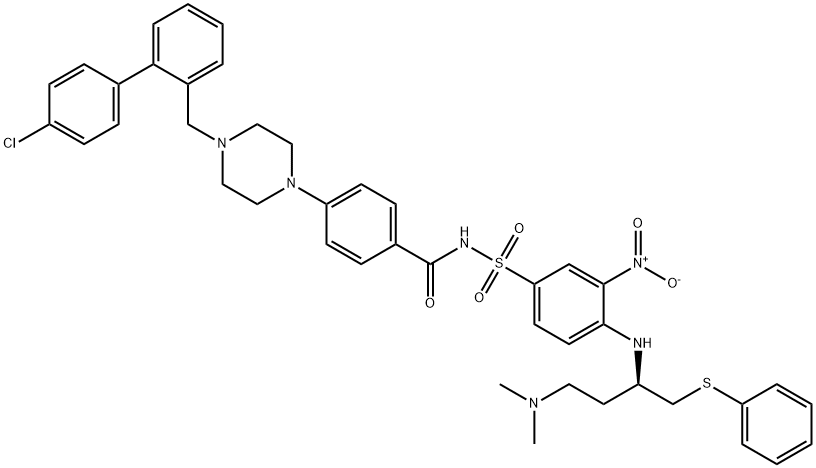
ABT-737 is a small-molecule inhibitor of the anti-apoptotic proteins Bcl-2, Bcl-X(L) and Bcl-w, with potential pro-apoptotic and antineoplastic activities, with an affinity two to three orders of magnitude more potent than previously reported compounds but no affinity towards less homologous proteins, such as BCL-B, MCL-1, and A1. Mechanistic studies reveal that ABT-737 does not directly initiate the apoptotic process, but enhances the effects of death signals, displaying synergistic cytotoxicity with chemotherapeutics and radiation. ABT-737 exhibits single-agent-mechanism-based killing of cells from lymphoma and small-cell lung carcinoma lines, as well as primary patient-derived cells, and in animal models, ABT-737 improves survival, causes regression of established tumors, and produces cures in a high percentage of the mice. ABT-737 binds to Bcl-2, Bcl-xL and Bcl-w with very high affinities (Ki <1 nM) and also shows a very high specificity over Mcl-1 and A1.
ABT-737 induces apoptosis in MM cells, including those resistant to conventional therapy. Importantly, ABT-737 decreases the viability of bortezomib-, dexamethasone-(Dex) and thalidomide-refractory patient MM cells. Additionally, ABT-737 abrogates MM cell growth triggered by interleukin-6 or insulin-like growth factor-1. Mechanistic studies show that ABT-737-induced apoptosis is associated with activation of caspase-8, caspase-9 and caspase-3, followed by poly(ADP-ribose) polymerase cleavage.
ABT-737 has shown single-agent activity against lymphoma and small-cell lung cancer as well as substantial antimyeloma activity both in vitro and in vivo. In recent studies, acute myeloid leukemia blast, origenitor, and stem cells are effectively killed by ABT-737 with normal hematopoietic cells intact. The disruption of the BCL-2/BAX complex and BAK-dependent but BIM-independent activation of the intrinsic apoptotic pathway could also be induced by ABT-737.