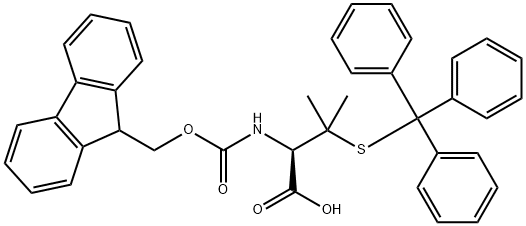
Fmoc-S-trityl-L-penicillamine is a coordination compound that contains a thiolate and amide group. It has been used as a model system for studying the interaction between proteins and metal ions, with the cyclic structure mimicking the active site of enzymes. The coordination of Fmoc-S-trityl-L-penicillamine to proteins is affected by trypsin, an enzyme that cleaves peptides at carboxyl side chains. Trypsin can also lead to dehydration of Fmoc-S-trityl-L-penicillamine, forming an eliminations product. This compound also reacts with lysine residues in proteins, resulting in an alkene byproduct that can be removed by hydrogenation.