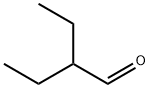
2-Ethylbutyraldehyde
- английское имя2-ETHYLBUTYRALDEHYDE
- CAS №97-96-1
- CBNumberCB9230646
- ФормулаC6H12O
- мольный вес100.16
- EINECS202-623-5
- номер MDLMFCD00006985
- файл Mol97-96-1.mol
| Температура плавления | -89°C |
| Температура кипения | 117 °C(lit.) |
| плотность | 0.814 g/mL at 25 °C(lit.) |
| показатель преломления | n |
| FEMA | 2426 | 2-ETHYLBUTYRALDEHYDE |
| Fp | 70 °F |
| температура хранения | Flammables area |
| форма | clear liquid |
| цвет | Colorless to Light yellow |
| Запах | at 10.00 % in dipropylene glycol. sweet green ethereal fruity cocoa |
| Odor Type | green |
| Растворимость в воде | Soluble in ether and alcohols. Slightly soluble in water. |
| Чувствительный | Air Sensitive |
| Номер JECFA | 256 |
| БРН | 1209330 |
| Стабильность | Stability Stable, but air sensitive. Flammable. Readily forms explosive mixtures with air. Incompatible with strong bases, strong reducing agents, oxidizing agents. |
| LogP | 1.73 |
| Справочник по базе данных CAS | 97-96-1(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | 2-ETHYLBUTYRALDEHYDE |
| FDA 21 CFR | 172.515 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 676JY5569P |
| Система регистрации веществ EPA | Butanal, 2-ethyl- (97-96-1) |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
| Коды опасности | F,Xi | |||||||||
| Заявления о рисках | 11-36/37/38 | |||||||||
| Заявления о безопасности | 16-26-36 | |||||||||
| РИДАДР | UN 1178 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ES2625000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29121900 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
2-Ethylbutyraldehyde химические свойства, назначение, производство
Химические свойства
2-Ethylbutyraldehyde has a pungent odor.Вхождение
Reported found in melon, French fried potato, wheaten bread, scallops, citrus fruits, white bread and maize.Использование
2-Ethylbutyraldehyde has been used in the preparation of aldoximes using aqueous hydroxylamine.Общее описание
A clear colorless liquid. Flash point 70°F. Less dense than water and insoluble in water. Vapors heavier than air.Реакции воздуха и воды
Highly flammable. With air slowly form peroxides. Insoluble in water.Профиль реактивности
2-ETHYLBUTYRALDEHYDE is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation.Опасность
Irritant to eyes and skin. Flammable, dangerous fire risk.Угроза здоровью
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.Пожароопасность
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.Профиль безопасности
Moderately toxic by ingestion. Mildly toxic by inhalation. A skin irritant. Flammable liquid. Can react vigorously with oxidizing materials. To fight fire, use alcohol foam, Co2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES.Возможный контакт
Used in organic synthesis of pharmaceuticals and rubber chemicals.Перевозки
UN1178 2-Ethyl butyraldehyde, Hazard Class: 3; Labels: 3-Flammable liquidНесовместимости
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, and reducing agents. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidationУтилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.2-Ethylbutyraldehyde запасные части и сырье
2-Ethylbutyraldehyde поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| 0519-86626038 | CHINA | 810 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +8617735180244 | CHINA | 4017 | 58 |