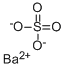
Барит (Ba (SO4))
- английское имяBarite
- CAS №13462-86-7
- CBNumberCB8881195
- ФормулаBaO4S
- мольный вес233.3896
- EINECS236-664-5
- файл Mol13462-86-7.mol
химическое свойство
| плотность | 4.300 g/cm3 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 25BB7EKE2E |
| Система регистрации веществ EPA | Barite (13462-86-7) |
Барит (Ba (SO4)) химические свойства, назначение, производство
Химические свойства
Barite is the primary, naturally occurring, barium-based mineral.Barium, atomic number 56, derives its name from Greek and means heavy. Barite is also known as baryte, and in Missouri is known as "tiff”. The primary countries in which commercial deposits of barite are currently found are the United States, China, India and Morocco. Barite’s high density and chemical inertness make it an ideal mineral for many applications.The chemical formula for barite is BaSO4. It has a high specific gravity of 4.50 g/cm3. Its Mohs hardness is 3.0 to 3.5. Barite, which may be found in a variety of colors including yellow, brown, white, blue, gray, or even colorless, typically has a vitreous to pearly luster.
Barite may be found in conjunction with both metallic and nonmetallic mineral deposits. To be economically viable for extraction, barite usually needs to be the predominant material in a deposit. The types of deposits in which it is normally found include vein, residual, and bedded. Vein and residual deposits are of hydrothermal origin, while bedded deposits are sedimentary.
Вхождение
Barite is a frequent fluorescent,most commonly displaying a not very saturated or intense light yellow color. Barite is found as beautiful and showy crystals in several places in South Dakota, particularly in the area of the Cheyenne and Moreau rivers.The barite crystals are tan to brown, glassy and transparent. Under short wave ultraviolet, these barites fluoresce butter yellow.Often there is a phosphorescence, usually a strong green-white.Accompanying calcite also fluoresces yellow, but more orange than the barite, and the combination of barite and calcite makes an attractive fluorescent combination.Barite is found as white to tan flat crystals standing on fluorite from the Buckskin Mountains, Yuma County, Arizona. This barite fluoresces orange-tan under long wave ultraviolet,while the fluorite responds with its usual blue,forming another attractive two-color fluorescent combination.Barite is also found at Bingham, New Mexico, as thick,white, blady crystals with fluorite. In these specimens, the barite fluoresces deep pink or weak red while the fluorite fluoresces blue,under long wave. Weak red fluorescing barite with fluorescing fluorite is also found at Elmwood, Tennessee.
Использование
Barite also is used as a filler, extender, or weighting agent in products such as paints, plastics, and rubber. Some specific applications include use in automobile brake and clutch pads, automobile paint primer for metal protection and gloss, use as a weighting agent in rubber, and in the cement jacket around underwater petroleum pipelines. In the metal-casting industry, barite is part of the mold-release compounds. Because barite significantly blocks x-ray and gamma-ray emissions, it is used as aggregate in high-density concrete for radiation shielding around x-ray units in hospitals, nuclear powerplants, and university nuclear research facilities. Ultrapure barite is used as a contrast medium in x-ray and computed tomography examinations of the gastrointestinal tract.Определение
barium sulphate: An insolublewhite solid, BaSO4, that occurs naturallyas the mineral barytes (orheavy spar) and can be prepared as aprecipitate by adding sulphuric acidto barium chloride solution; r.d. 4.50;m.p. 1580°C. The rhombic formchanges to a monoclinic form at1149°C. It is used as a raw materialfor making other barium salts, as apigment extender in surface coatingmaterials (called blanc fixe), andin the glass and rubber industries.Barium compounds are opaque toX-rays, and a suspension of the sulphatein water is used in medicine toprovide a contrast medium for X-raysof the stomach and intestine. Althoughbarium compounds are extremelypoisonous, the sulphate issafe to use because it is very insoluble.Барит (Ba (SO4)) поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | |
| +8615350851019 | China | 1001 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +8619521488211 | China | 2558 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +8613367258412 | China | 10319 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +86-86-13583358881 +8618560316533 |
China | 3094 | 58 | |
| China | 12341 | 58 |