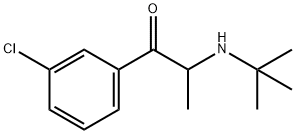
Бупропион
- английское имяBupropion
- CAS №34911-55-2
- CBNumberCB8140067
- ФормулаC13H18ClNO
- мольный вес239.74
- номер MDLMFCD00055209
- файл Mol34911-55-2.mol
химическое свойство
| Температура плавления | 233-234°C |
| Температура кипения | bp.005 52° |
| плотность | 1.066±0.06 g/cm3(Predicted) |
| растворимость | ethanol: 193 mg/mL solutions may be stored for several days at 4 °C. |
| форма | solid |
| пка | pKa 7.0 (Uncertain) |
| цвет | white |
| BCS Class | 1 |
| Справочник по базе данных CAS | 34911-55-2(CAS DataBase Reference) |
| FDA UNII | 01ZG3TPX31 |
| Словарь наркотиков NCI | Amfebutamone |
| Код УВД | N06AX12 |
| Система регистрации веществ EPA | 1-Propanone, 1-(3-chlorophenyl)-2-[(1,1-dimethylethyl)amino]- (34911-55-2) |
| Коды опасности | Xn |
| Заявления о рисках | 22 |
| Заявления о безопасности | 36 |
| WGK Германия | 3 |
| RTECS | UG8858000 |
| Класс опасности | IRRITANT |
| Банк данных об опасных веществах | 34911-55-2(Hazardous Substances Data) |
Бупропион химические свойства, назначение, производство
Описание
Bupropion hydrochloride, an aminoketone structurally unrelated to tricyclics or tetracyclics, is a dopamine uptake blocker with antidepressant activity. Its clinical efficacy is reportedly comparable to that of amitriptyline, yet unlike most conventional antidepressants, bupropion hydrochloride is not associated with orthostatic hypotension or other cardiovascular side-effects.Использование
vasoconstrictor;non-selective agonist of all adrenergic receptorsБиологические функции
Bupropion (Wellbutrin) is a pharmacologically unique antidepressant, since it is a weak inhibitor of both dopamine and norepinephrine neuronal reuptake. However, its actual antidepressant activity is not well understood. Bupropion is generally well tolerated and does not block muscarinic, histaminergic, or adrenergic receptors. Unlike the SSRIs and venlafaxine, bupropion does not cause sexual side effects. However, it can cause CNS stimulation, including restlessness and insomnia. High doses of bupropion, given as its original formulation, were associated with a risk of seizures in 0.4% of patients. However, this risk is lower with slow-release bupropion (Wellbutrin SR). This formulation still requires dosing twice a day, and bupropion is contraindicated in patients with a history of seizures. Bupropion inhibits the cytochrome P450 2D6 isoenzyme and may elevate blood levels of drugs metabolized by this route.Общее описание
The mechanism of action of bupropion (Wellbutrin) is consideredcomplex and reportedly involves a block of DA reuptakevia the dopamine transporter (DAT), but the overallantidepressant action is noradrenergic. A metabolite thatcontributes to the overall action and its formation can beeasily rationalized. Oxidation of one of the methyl groupson the t-butyl substituent yields hydroxybupropion, an activemetabolite. Reduction of the keto group also occurs,yielding threohydrobupropion and erythrohydrobupropion.Both of these metabolites are also active.Hydroxybupropion is half as potent as the parent bupropion,and the hydrobupropion isomers are five times less potent.The presence of these metabolites, especially hydroxybupropionwhich is formed by cytochrome P450 2D6(CYP2D6), suggests that there will be a myriad of drug interactionswith bupropion.
Фармаколо?гия
Bupropion is an α-aminoketone that is structurally related to amphetamines, and it exhibits unique activity comparable to that of other antidepressants. It is believed that bupropion restores the total amount of norepinephrine in the body. This compound is a poor reuptake inhibitor of dopamine, and does not exhibit anticholinergic activity or inhibit MAO. Its efficacy as an antidepressant is comparable to that of tricyclic antidepressants, and as a serotonin uptake inhibitor it is comparable to fluoxetine.