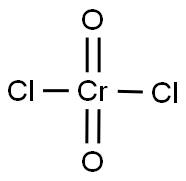
Chromyl chloride
- русский язык имя
- английское имяChromyl chloride
- CAS №14977-61-8
- CBNumberCB7853128
- ФормулаCl2CrO2
- мольный вес154.9
- EINECS239-056-8
- номер MDLMFCD00010951
- файл Mol14977-61-8.mol
| Температура плавления | -96.5 °C (lit.) |
| Температура кипения | 117 °C (lit.) |
| плотность | 1.911 g/mL at 25 °C (lit.) |
| Fp | 117°C |
| растворимость | reacts with H2O; soluble in ctc, chloroform,benzene |
| форма | liquid |
| Удельный вес | 1.911 |
| цвет | red |
| Растворимость в воде | Soluble in carbon tetrachloride, carbon disulfide, benzene, nitrobenzene, chloroform, and POCL3. Insoluble in water(Reacts). |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,2248 |
| Диэлектрическая постоянная | 2.6(20℃) |
| Пределы воздействия | ACGIH: TWA 0.0001 ppm; STEL 0.00025 ppm (Skin) NIOSH: TWA 0.0002 mg/m3 |
| Справочник по базе данных CAS | 14977-61-8(CAS DataBase Reference) |
| FDA UNII | JQU316FZ5W |
| Система регистрации веществ EPA | Chromium(VI) dioxychloride (14977-61-8) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
| Коды опасности | O,T,C,N | |||||||||
| Заявления о рисках | 49-46-8-35-43-50/53-34-23/24/25-45 | |||||||||
| Заявления о безопасности | 53-45-60-61-36/37/39-27-26-17 | |||||||||
| РИДАДР | UN 3244 8/PG 2 | |||||||||
| OEB | E | |||||||||
| OEL | TWA: 0.001 mg Cr(VI)/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GB5775000 | |||||||||
| F | 8-10-21 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | I | |||||||||
| кода HS | 2827499090 | |||||||||
| Банк данных об опасных веществах | 14977-61-8(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H350:Может вызывать раковые заболевания.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H340:Может вызывать генетические дефекты.
H271:Сильный окислитель; может вызвать возгорание или взрыв.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Chromyl chloride химические свойства, назначение, производство
Химические свойства
Chromyl chloride is a dark red fuming liquid with a musty, burning odor.Физические свойства
Dark red, fuming liquid; reddish yellow vapors; musty buring odor; density 1.91 g/mL; freezes at -96.5°C; boils at 117°C; reacts with water; soluble in chloroform, carbon tetrachloride, benzene, carbon disulfide and nitrobenzene.Использование
Highly spin-polarized chromium dioxide (CrO2) thin films were deposited on 100 TiO2 substrates by chemical vapor deposition using chromyl chloride as a precursor. Chromyl chloride (Cr02C12) reacts with cyclohexane solvent at 75 OC to give a dark precipitate along with chlorocyclohexane and a small amount of cyclohexene (in 10.0 and ca. 0.3% yields based on chromium). Chromyl chloride, CrO2C12, oxidizes cyclooctane, isobutane, and toluene under mild conditions (25-60" C). The reactions give chlorinated products (chlorocyclooctane, tert- butyl chloride, and benzyl chloride) and a dark chromium-containing precipitate. Used for Organic oxidations and chlorination, chromium coordination complexes, catalyst for polymerization of olefins. Chromyl chloride in carbon tetrachloride solution reacts in the cold with cyclohexene, cyclopentene, and 1-hexene to give the various isomeric chlorohydrins as the major products.Подготовка
Chromyl chloride is prepared by reacting chromium(III) chloride with hydrochloric acid:CrO3 + 2HCl → CrO2Cl2 + H2O
Also, it may be prepared by warming potassium dichromate with potassium chloride in concentrated sulfuric acid:
K2Cr2O7 + 4KCl + 3H2SO4 → 2Cr2O2Cl2 + 3K2SO4 + 3H2O.
Определение
A dark red covalent liquid prepared either by distilling a dry mixture of potassium dichromate and sodium chloride with concentrated sulfuric acid or by the action of concentrated sulfuric acid on chromium(VI) oxide dissolved in concentrated hydrochloric acid. Chromyl chloride is hydrolyzed by water, and with solutions of alkalis it undergoes immediate hydrolysis to produce chromate ions. It is used as an oxidizing agent in organic chemistry. Chromyl chloride oxidizes methyl groups at the ends of aromatic side chains to aldehyde groupings (étard’s reaction).Общее описание
A dark red fuming liquid with a pungent odor. Corrosive to tissue.Профиль реактивности
Chromyl chloride is a powerful and often violent oxidizing agent. Reacts readily with many inorganic and organic materials in the absence of a dilutent. Contact with hydrogen sulfide or phosphine can cause ignition. Contact with phosphorus tribromide, acetone, ethanol, ether, and turpentine causes ignition. Contact with moist phosphorus or with phosphorus trichloride leads to explosive reaction. Contact with ammonia causes incandescence. Reacts with sodium azide to form chromyl azide, which is explosive in the absence of a dilutent. Causes ignition of flowers of sulfur and of urea on contact. [Bretherick, 1979, p. 822-823].Опасность
Corrosive to tissue. Strong oxidizing agent. Skin and upper respiratory tract irritant. Probable carcinogen.Угроза здоровью
Inhalation causes severe irritation of upper respiratory system. Contact with eyes or skin causes irritation and burning. Ingestion causes burning of mouth and stomach.Пожароопасность
Behavior in Fire: Vapors are very irritating to eyes and mucous membranes. May increase severity of fire.Профиль безопасности
Suspected carcinogen. Probably a poison by various routes. Mutation data reported. Corrosive. A strong irritant. Hydrolyzes to form chromic and hydrochloric acids. A strong oxidner and chlorinating agent. Violent reaction with water. Reacts violently with alcohol, ether, acetone, turpentine. Ignites or explodes on contact with nonmetal halides (e.g., disulfur dichloride, phosphorus trichloride, and phosphorus tribromide), nonmetal hydrides (e.g., hydrogen sulfide and hydrogen phosphide), flowers of sulfur, moist phosphorus, sodium azide, and urea. During preparation can violently explode. Incompatible with ammonia, disulfur dichloride, organic solvents, phosphorus, phosphorus trichloride, sodium azide, and sulfur. When heated to decomposition it emits toxic fumes of Cl-. See also CHROMIUM COMPOUNDS.Возможный контакт
Chromium oxychloride is used in making chromium complexes and dyes; and used in various organic oxidation and chlorination reactionsПеревозки
UN1758 Chromium oxychloride, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Purify it by distillation under reduced pressure. It hydrolyses violently with H2O and is a powerful oxidant which explodes with P, and ignites in contact with S, NH3, EtOH and many organic compounds. TOXIC.Несовместимости
Contact with water is violent and forms hydrochloric and chromic acids, and chlorine gas. A powerful oxidizer. Reacts violently with acetone, alcohol, ammonia, ether, fuels, organic solvents, moist phosphorus, phosphorus trichloride; sodium azide; sulfur, reducing agents; turpentine. Contact with nonmetal halides, such as disulfur dichloride, phosphorus trichloride; and phosphorus tribromide; nonmetal hydrides, such as hydrogen sulfide; hydrogen phosphide, and urea, causes a danger fire and explosion hazardChromyl chloride запасные части и сырье
Chromyl chloride поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-29-87569262 +86-15003564040 |
China | 3167 | 58 | |
| 18503026267 | CHINA | 9636 | 58 | |
| United States | 2344 | 58 | ||
| +86-89586680 +86-13289823923 |
China | 8670 | 58 | |
| +1-+1(833)-552-7181 | United States | 52924 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 021-61259108 18621169109 |
China | 40228 | 62 |