Палладий(II) хлорид
- английское имяPalladium chloride
- CAS №7647-10-1
- CBNumberCB7222436
- ФормулаCl2Pd
- мольный вес177.33
- EINECS231-596-2
- номер MDLMFCD00003558
- файл Mol7647-10-1.mol
| Температура плавления | 678-680 °C(lit.) | ||||||||||||||
| плотность | 4 g/mL at 25 °C(lit.) | ||||||||||||||
| давление пара | 0Pa at 20℃ | ||||||||||||||
| температура хранения | Store below +30°C. | ||||||||||||||
| растворимость | 55.6g/l insoluble | ||||||||||||||
| форма | Powder/Solid | ||||||||||||||
| цвет | Yellow | ||||||||||||||
| Удельный вес | 4 | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| РН | 2.15 (30g/l, H2O, 20℃) | ||||||||||||||
| Растворимость в воде | Insoluble | ||||||||||||||
| Разложение | 200 °C | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Мерк | 14,6990 | ||||||||||||||
| Space group | Pnmn | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Стабильность | Stable. Incompatible with strong oxidizing agents. | ||||||||||||||
| Справочник по базе данных CAS | 7647-10-1(CAS DataBase Reference) | ||||||||||||||
| FDA UNII | N9214IR8N7 | ||||||||||||||
| Система регистрации веществ EPA | Palladium dichloride (7647-10-1) | ||||||||||||||
| UNSPSC Code | 12352302 | ||||||||||||||
| NACRES | NA.23 |
| Коды опасности | C,Xi,T+,T,Xn | |||||||||
| Заявления о рисках | 34-43-40-28-41-37/38-25-22 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-37/39-28-27-36/37-27/28 | |||||||||
| РИДАДР | UN 1789 8/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | RT3500000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28439090 | |||||||||
| Банк данных об опасных веществах | 7647-10-1(Hazardous Substances Data) | |||||||||
| Токсичность | MLD i.v. in rabbits: 0.0186 g/kg (Orestano) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Палладий(II) хлорид химические свойства, назначение, производство
Описание
Palladium chloride is a commonly used precious metal catalysts, molecular formula is PaCl2, the appearance is brown-red needle-like crystals or powder, easily deliquescence, the relative density is 4.0 (18 ℃), melting point is 500 ℃ (decomposition), soluble in water, ethanol, acetone and hydrogen bromide. Decomposition in ammonia chloride, potassium iodide, ammonia solution, and precipitation of palladium.[Uses]
(1)used as the analysis reagents, such as determination of trace palladium, mercury, thallium, iodine, etc.
(2) palladium test strips is used to test carbon monoxide.
(3) also used to search for cracks of buried underground gas pipeline cracks, study of agricultural plant resources, preparation of palladium catalyst, electroplating watch parts and photography, and so on.
[Preparation method] by melting palladium dichloride hydrate, make it lost part of chloride to get Palladium chlorine finished products.
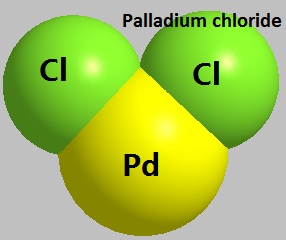
Figure 1 the molecular structure of Palladium chloride.
Химические свойства
Palladium chloride is a dark brown powder, hygroscopic (absorbs moisture from the air). It is incompatible with acids, aluminium, ammonia, magnesium, nitrates, zinc, heat, thiocyanates, and organic solvents. Thermal decomposition of palladium chloride may release chlorine, hydrogen chloride, and oxides of palladium. It is used as a catalyst, photographic, and electroplating reagent. Palladium and its alloys are used as catalysts in the (petro)chemical and, above all, in the automotive industries. Applications of palladium compounds for electronics and electrical technology include use in metallisation processes (thick film paste), electrical contacts and switching systems, in the synthesis of semiconducting metal-containing polymers in which the polypyrrole backbone has a conformational energy minimum and is nearly planar. Palladium chloride is a stable chemical substance and is incompatible with strong oxidising agents.Физические свойства
l Properties Red rhombohedral crystal; hygroscopic; density 4.0g/cm3; melts at 679°C; dissolves slowly in water; also soluble in ethanol and acetone; dissolves rapidly in hydrochloric acid.Использование
Palladium chloride is used in photography; toning solutions; electroplating parts of docks and watches; detecting carbon monoxide leaks in buried gas pipes; manufacture of indelible ink; preparation of metal for use as a catalyst; catalyst in jewelry; in dental alloys.Подготовка
Palladium dichloride is prepared by dissolving palladium metal in aqua regia or hydrochloric acid in the presence of chlorine. Alternatively, it may be prepared by heating palladium sponge with chlorine gas at 500°C.Методы производства
The palladium powder is added to the reactor containing hydrochloric acid, with stirring, passing the air, an oxidation reaction is performed, generating palladium chloride solution, the solution is purified, filtered, concentrated by evaporation, cooling and crystallization, centrifugal separation, and dried to obtain a palladium chloride finished products.Pd+2HCl+0.5O2→PdCl2+H2O
Реакции
Palladium chloride dissolves in HCl forming tetrachloropalladate ion,PdCl2+2Cl¯→ [PdCl4]2¯
The complex ion catalyzes various types of organic reactions including oxidation of ethylene to acetaldehyde in aqueous solution (the Wacker Process):
PdCl42¯+ C2H4 + H2O → CH3CHO + Pd + 2HCl + 2Cl¯
Palladium dichloride forms polymeric carbonyl complexes when the dry chloride is heated in a stream of carbon monoxide charged with methane vapor. Such complexes include [PdCl2(CO)n] and [PdCl(CO)2]n. The reaction also occurs in aqueous phase resulting in decolorization of the solution.
When H2S is passed through palladium dichloride solution, it yields a brown-black precipitate of palladium monosulfide, PdS.
When heated with sulfur at 450 to 500°C, palladium dichloride forms palladium disulfide, PdS2, a grey-black crystalline compound, insoluble in strong acids but soluble in aqua regia, and which converts to monosulfide, PdS, on heating at 600°C.
When ammonia gas is passed through an aqueous solution of PdCl2, the product is tetrammine palladium(II) chloride, Pd(NH4)2Cl2. The same product also is obtained in dry state by passing ammonia gas over anhydrous Palladium chloride.
Общее описание
Dark brown crystals.Реакции воздуха и воды
Deliquescent. Water soluble.Профиль реактивности
Palladium chloride is a weak oxidizing agent. Palladium chloride is reduced in solution by hydrogen or carbon monoxide to metallic palladium. . Decomposed at high temperatures to metallic palladium and chlorine.Пожароопасность
Flash point data for Palladium chloride are not available. Palladium chloride is probably combustible.Профиль безопасности
Poison by intraperitoneat, intravenous, and intratracheal routes. Moderately toxic by ingestion. Experimental reproductive effects. A skin irritant. Questionable carcinogen with experimental carcinogenic data. Human mutation data reported. When heated to decomposition it emits highly toxic fumes of Cl-. See also PALLADIUMМетоды очистки
The anhydrous salt is insoluble in H2O and dissolves in HCl with difficulty. The dihydrate forms red hygroscopic crystals that are readily reduced to Pd. Dissolve it in conc HCl through which dry Cl2 is bubbled. Filter this solution which contains H2PdCl4 and H2PdCl6 and on evaporation it yields a residue of pure PdCl2. [Grube in Handbook of Preparative Inorganic Chemistry (Ed Brauer) Academic Press Vol II p 1582 1965, Mozingo Org Synth Coll Vol III 685 1955.]Палладий(II) хлорид запасные части и сырье
сырьё
1of2
запасной предмет
- н-бутил винилового эфира
- 3-пирид-4-илбензойная кислота
- 1,1'-бис(ди-трет-бутилфосфино)ферроцен палладий дихлорид в компании Реарус
- 6-гидроксииндол
- 3-ЦИАНО-6-МЕТИЛ (1H) ИНДАЗОЛ
- Палладий (II) ацета
- 1,1'-бис(дифенилфосфино)ферроцен-палладий(II)дихлорид дихлорметан аддукт в компании Реарус
- N,N,N',N'-Тетраметил-2-бутен-1,4-диамин
- 6-бензилоксииндол
- Трис(дибензилиденацетон)дипалладий-хлорформ аддук
- 6-МЕТИЛ-3-(1H)ИНДАЗОЛКАРБОНОВАЯ КИСЛОТА
- 2-хлор-3-оксопентилацетат
- palladium catalyst supported by poly (2, 6-dimethyl-1, 4-phenylene oxide)
- [1,1'-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)
- Далтробан
- Дихлорбис(трициклогексилфосфин)палладий(II)
- Бис(бензонитрил)палладий(II) хлорид
- Палладий(II) ацетилацетонат
- ПРОПИОНОВЫЙ АЛЬДЕГИД
- Бис(дибензилиденацетон)палладий
- Дихлор [1,2-бис (дифенилфосфино) этан] палладий (II)
- N-(4-THIOPHEN-2-YL-PHENYL)-ACETAMIDE
- Dichloro(1,5-cyclooctadiene)palladium(II)
- Салбутамол гемисульфат
- Тетракис (трифенилфосфин) палладий
- 3-aminopropanamide
- Бис(три-трет-бутилфосфин)палладий(0)
- Дифенилацетилен
- 3-аминобензойная кислота
- 4-(3-тиенил)бензальдегид
1of8
Палладий(II) хлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-029-86333380 18829239519 |
China | 899 | 58 | ||
| +1-+1(833)-552-7181 | United States | 52924 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 |