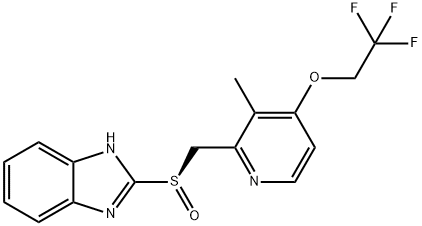
R-(+)-Lansoprazole
- русский язык имя
- английское имяR-(+)-Lansoprazole
- CAS №138530-94-6
- CBNumberCB71269821
- ФормулаC16H14F3N3O2S
- мольный вес369.36
- EINECS1308068-626-2
- номер MDLMFCD13196699
- файл Mol138530-94-6.mol
| Температура плавления | 66-68?C |
| Температура кипения | 555.8±60.0 °C(Predicted) |
| плотность | 1.50±0.1 g/cm3(Predicted) |
| температура хранения | Sealed in dry,2-8°C |
| растворимость | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| форма | Solid |
| пка | 9.56±0.10(Predicted) |
| цвет | Off-White to Dark Brown |
| оптическая активность | [α]/D +142 to +158°, c =1 in chloroform-d |
| Стабильность | Hygroscopic |
| FDA UNII | UYE4T5I70X |
| Код УВД | A02BC06 |
| UNSPSC Code | 12352200 |
| NACRES | NA.77 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P272:Не уносить загрязненную спецодежду с места работы.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P314:В случае плохого самочувствия обратиться к врачу.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
R-(+)-Lansoprazole химические свойства, назначение, производство
Описание
The mechanism of PPIs involves the irreversible binding to the hydrogen/potassium adenosine triphosphatase enzyme system, commonly referred to as the gastric proton pump, of the gastric parietal cell. As the last stage in gastric acid secretion, blockade of the gastric proton pump is an effective treatment for a variety of diseases requiring acid suppression, such as heartburn, peptic ulcers, and GERD. Dexlansoprazole is the latest PPI to hit the market, joining the ranks of omeprazole, rabeprazole, pantoprazole, esomeprazole, and lansoprazole, and is the Renantiomer of the racemic lansoprazole. Compared to its predecessors, dexlansoprazole exhibits improved pharmacokinetics with slower clearance and longer terminal half-life. In addition, dexlansoprazole utilizes a novel DDR technology; drug release is optimized through the use of granules with different pH-dependent dissolution profiles, thereby providing an initial release in the proximal small intestine within 1-2 h of administration followed by a subsequent release at distal regions of the small intestine several hours later. With its longer duration of action culminating in more effective acid suppression, dexlansoprazole may have an advantage over conventional PPIs that possess single release formulations (immediate or delayed). Similar to all PPIs, dexlansoprazole is a prodrug that consists of pyridine and benzimidazole rings with a latent sulfenamide moiety. In order to form the disulfide bond with cysteine residues of the proton pump, dexlansoprazole must be activated through two protonations followed by a spontaneous rearrangement to unmask the sulfenamide.Химические свойства
Brown SolidИспользование
The R-enantiomer of Lansoprazole; a gastric proton pump inhibitor. An antiulcerativeКлиническое использование
Takeda Pharmaceuticals received approval of dexlansoprazole, a dual release formulation of the (R)-isomer of lansoprazol proton pump inhibitor (PPI) already in the market, from the FDA in January 2009. Dexlansoprazole is a delayed release capsule for the oncedaily, oral treatment of heartburn associated with symptomatic non-erosive gastroesophageal reflux disease (GERD), the healing of erosive esophagitis (EE) and the maintenance of healed EE. The dual release formulation is designed to provide two separate releases of medication, one at 1–2 h and then another at 4–5 h after treatment, for extended efficacy in the treatment of GERD.Побочные эффекты
The most commonly recorded adverse reactions that occurred at a higher incidence than placebo were diarrhea, abdominal pain, nausea, vomiting, flatulence, and upper respiratory tract infection. As dexlansoprazole inhibits gastric acid secretion, its use is expected to interfere with the absorption of drugs with pH-dependent oral bioavailability. Since the HIV protease inhibitor atazanavir is dependent on gastric acid for absorption, dexlansoprazole should not be co-administered with atazanavir to avoid a loss of therapeutic efficacy. While co-administration of dexlansoprazole did not affect the pharmacokinetics of warfarin or INR (international normalized ratio: the ratio of a patient s prothrombin time to a normal sample), there have been reports of increased INR and prothrombin time in patients receiving concomitant treatment with PPIs and warfarin. Since increases in INR and prothrombin time may lead to abnormal bleeding and possibly death, concomitant use of dexlansoprazole and warfarin may necessitate monitoring for increases in INR and prothrombin time.R-(+)-Lansoprazole запасные части и сырье
R-(+)-Lansoprazole поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-18600796368 +86-18600796368 |
China | 484 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +17326109938 | CHINA | 174 | 58 | |
| 86-714-3992388 | United States | 14332 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 |