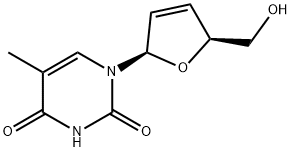
Ставудин
- английское имяStavudine
- CAS №3056-17-5
- CBNumberCB4450928
- ФормулаC10H12N2O4
- мольный вес224.21
- EINECS641-374-0
- номер MDLMFCD00132921
- файл Mol3056-17-5.mol
| Температура плавления | 159-160°C |
| альфа | D25 -39.4° (c = 0.701 in water); D20 -46.1° (c = 0.7 in water) |
| плотность | 1.374±0.06 g/cm3(Predicted) |
| показатель преломления | -46 ° (C=0.69, H2O) |
| температура хранения | -20°C |
| растворимость | Soluble in water, sparingly soluble in ethanol (96 per cent), slightly soluble in methylene chloride. It shows polymorphism (5.9). |
| форма | Solid |
| пка | 9.47±0.10(Predicted) |
| цвет | White |
| Растворимость в воде | 5-10 g/100 mL at 21 ºC |
| BCS Class | 1,3 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | XNKLLVCARDGLGL-JGVFFNPUSA-N |
| Справочник по базе данных CAS | 3056-17-5(CAS DataBase Reference) |
| Словарь онкологических терминов NCI | stavudine |
| FDA UNII | BO9LE4QFZF |
| Словарь наркотиков NCI | stavudine |
| Код УВД | J05AF04 |
| Система регистрации веществ EPA | Thymidine, 2',3'-didehydro-3'-deoxy- (3056-17-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.77 |
| Коды опасности | Xi |
| Заявления о рисках | 36/37/38 |
| Заявления о безопасности | 26-36 |
| WGK Германия | 2 |
| RTECS | XP2075000 |
| кода HS | 29349990 |
| Банк данных об опасных веществах | 3056-17-5(Hazardous Substances Data) |
| Токсичность | LD50 oral in rat: 4gm/kg |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H341:Предполагается, что данное вещество вызывает генетические дефекты.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Ставудин химические свойства, назначение, производство
Описание
Stavudine, a dideoxynucleoside analog of thymidine, has been introduced in the U.S.A. for the treatment of late-stage AIDS patients who are refractory to other AIDS treatments. Similar as other currently available agents for AIDS treatment such as zidovudine (AZT), didanosine, and zalcitabine, the anti-HIV activity of these 2'3'- dideoxynucleosides is ascribed to the inhibitory effect of their corresponding 5'- triphosphates against the HlVsncoded RNAdependent DNA polymerase (reverse transcriptase). While some of these drugs have rapid development of drug resistance, stavudine is active against AZT-resistant HIV strains. It has a favorable pharmacokinetic profile with more complete and less variable oral absorption than AZT and didanosine and has a bioavailability of 80-90%.Химические свойства
Colourless solidИспользование
Stavudine (Zidovudine EP Impurity A) is used as an antiviral. A reverse transcriptase inhibitor.Показания
Stavudine (d4T, Zerit) is a thymidine nucleoside analogue that is active against HIV-1 and HIV-2. It is approved for the therapy of HIV infection as part of a multidrug regimen and is also used for postexposure prophylaxis.Определение
ChEBI: A nucleoside analogue obtained by formal dehydration across positions 2 and 3 of thymidine. An inhibitor of HIV-1 reverse transcriptaseАнтимикробная активность
Stavudine is active against HIV-1, HIV-2 and HTLV-1.Приобретенная устойчивость
Resistance to stavudine is identical to that seen for zidovudine. Mutations at positions 41, 67 and 70, and positions 210, 215 and 219 (the ‘thymidine analog mutations’) of the reverse transcriptase genes are associated with diminished antiretroviral efficacy.Общее описание
Stavudine, 2'3'-didehydro-2'-deoxythymidine (D4T, Zerit), isan unsaturated pyrimidine nucleoside that is related to thymidine.The drug inhibits the replication of HIV by a mechanismsimilar to that of its close congener, AZT.Stavudine is bioactivatedby cellular enzymes to a triphosphate.Stavudine is available as capsules for oral administration.The drug is acid stable and well absorbed (about 90%) followingoral administration. Stavudine has a short half-life(1–2 hours) in plasma and is excreted largely unchanged(85%–90%) in the urine.As with ddC, the primary doselimitingeffect is peripheral neuropathy.
Stavudine isrecommended for the treatment of adults with advancedHIV infection who are intolerant of other approved therapiesor who have experienced clinical or immunological deteriorationwhile receiving these therapies.
Реакции воздуха и воды
Water soluble.Профиль реактивности
Stavudine is sensitive to heat. Incompatible with strong oxidizing agents .Опасность
Moderately toxic by ingestion.Пожароопасность
Literature sources indicate that Stavudine is combustible.Фармацевтические приложения
An analog of thymidine formulated for oral administration.Фармакокине?тика
Oral absorption: 86%Cmax 40 mg twice daily: 0.54 mg/L
Plasma half-life: 1.4 h
Volume of distribution: 0.66 L/kg
Plasma protein binding: <5%
Absorption and distribution
It is rapidly absorbed with or without food. CNS penetration is moderate. The estimated semen:plasma ratio is >1. It is secreted into breast milk.
Metabolism and excretion
The metabolic fate in humans has not been elucidated. Renal elimination accounts for approximately 40% of overall clearance at a rate almost twice that of endogenous creatinine, indicating glomerular filtration and active tubular secretion. Clearance decreases as creatinine clearance decreases and the dosage should be adjusted in patients with reduced renal function. Pharmacokinetics are not significantly altered in patients with hepatic impairment.
Клиническое использование
Treatment of HIV infection in adults and childrenПобочные эффекты
The adverse effects with which stavudine is most frequently associated are headache, diarrhea, skin rash, nausea, vomiting, insomnia, anorexia, myalgia, and weakness. Peripheral neuropathy consisting of numbness, tingling, or pain in the hands or feet is also common with higher doses of the drug. Significant elevation of hepatic enzymes may be seen in approximately 10 to 15% of patients. Lactic acidosis occurs more frequently with stavudine than with other NRTIs. Viral resistance to stavudine may develop, and cross-resistance to zidovudine and didanosine may occur.Меры предосторожности
Stavudine possesses several clinically significant interactionswith other drugs. Although hydroxyurea enhancesthe antiviral activity of stavudine and didanosine,combination therapy that includes stavudine anddidanosine, with or without hydroxyurea, increases therisk of pancreatitis. Combinations of stavudine and didanosineshould not be given to pregnant women becauseof the increased risk of metabolic acidosis.Zidovudine inhibits the phosphorylation of stavudine;thus, this combination should be avoided.Ставудин запасные части и сырье
сырьё
- Anhydrous pyridine
- Uridine, 5-methyl-, 2',3',5'-triacetate
- Uridine, 2',3'-dideoxy-2'-iodo-5-methyl-, 5'-(2,2-dimethylpropanoate)
- Thymidine, 2',3'-didehydro-3'-deoxy-5'-O-[(1,1-dimethylethyl)diphenylsilyl]- (9CI)
- ((2S,5R)-5-(2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl)-2,5-dihydrofuran-2-yl)methyl acetate
1of4
Ставудин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 86-714-3992388 | United States | 14332 | 58 | |
| +1-631-485-4226 | United States | 19553 | 58 |